Abstract
Pancreatic cancer (PC) is one of the most dangerous cancers with less than 5% survival rate in 5 years. This study was to evaluate the antitumor activities of dFv-LDP-AE and dFv-R-LDP-AE, two energized fusion protein targeting gelatinases, on pancreatic cancer. The fusion protein dFv-LDP-AE consists of two tandem anti-gelatianses scFv and an enediyne antibiotic lidamycin (LDM) for receptor binding and cell killing. To improve the penetration capability, the fusion protein dFv-LDP-AE was integrated with an arginine-rich cell penetrating peptide (Arg)9 and then generated the fusion protein dFv-R-LDP-AE. The current study demonstrated that dFv-LDP and dFv-R-LDP had high affinity with the antigen gelatinases and PC cells, the integration of (Arg)9 could increase the penetration rate of fusion protein in SW-1990 and PANC-1 cells. After enediyne-energized with chromophore of lidamycin, the energized fusion protein dFv-LDP-AE and dFv-R-LDP-AE showed potent cytotoxicity to PC cells and could induced the robust cell apoptosis and necrosis in vitro. Western blot showed that dFv-R-LDP-AE could increase PARP cleavage, and inhibited the expression of VEGF, Cyclin D1, Cox-2 and Bcl-2 in SW-1990 and PANC-1 cells. In vivo, at a tolerated dosage, dFv-LDP, dFv-LDP-AE and dFv-R-LDP-AE inhibited tumor growth by 20.42%, 56.31% (P < 0.01, compared to that of control) and 74.2% (P < 0.05, compared to that of dFv-LDP-AE) in pancreatic cancer SW-1990 xenografted mice, respectively. Moreover, the results of in vivo optical imaging showed that fusion protein dFv-R-LDP displayed prominent accumulation in the tumor in SW-1990 xenografted mice and Capan-2 orthotopic transplanted mice. These results showed that dFv-R-LDP-AE possessed potent antitumor efficacy on PC, which indicating it could be a promising candidate for targeting therapy of PC.
Keywords: Pancreatic cancer, gelatianse, lidamycin, arginine-rich cell penetrating peptide, optical imaging
Introduction
Pancreatic cancer (PC) is one of the malignancies which greatly threaten the health of human health, the prognosis of PC is depressed and the 5-year survival rate was lower than 5% 1. The debulking by surgery is the best way to treat PC, however, more than 80% patient with PC was in advanced stage and lost the opportunity of surgery, moreover, there is no very effective medicine for the treatment of PC. Gemcitabine, which used most commonly, only shows efficiency in 20% patient, therefore it is very imperative to develop novel agent for PC therapy in clinic 2.
Lidamycin (also named C-1027), an enediyne antibiotics, had shown potent antitumor efficacy in kinds of caner cell line in vitro and in vivo. However, like one sword has two sides, the systemic side effects of lidamycin also cannot been ignored. Antibody-drug conjugates already show its efficacy in cancer therapy in clinic, there are more than two antibody-drug conjugates already approved by US FDA until now. To improve the antitumor efficacy of lidamycin, antibody conjugated of lidamycin seems to be a reasonable approach to further improve the efficacy of lidamycin whereas lower the side effects after systemic intravenous injection, it was documented that antibody or its fragments conjugated with lidamycin showed improved antitumor efficacy as compared to lidamycin alone 3-5.
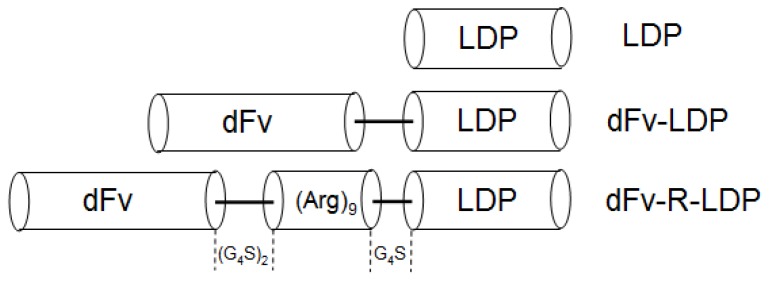
Gleatinases, including gelatinase A (MMP-2) and B (MMP-9), play an important role in the cancer invasion and metastasis. It was reported that gelatianses were related with the prognosis and microvessel density (MVD) in patients with pancreatic cancer, and would be a potential target for cancer therapy 6, 7. Antibody against gelatinases had been developed and the conjugation of scFv against gelatinases with lidamycin already showed potent antitumor efficacy in several kinds of cancer types, such as lung cancer, hepatoma 5, 8, 9. In this study, the antitumor efficacy of scFv-based fusion protein against gelatianses conjugated with lidamycin was investigated in pancreatic cancer. As the internalization of anticancer agent, especially the antibody-drug conjugates, is a very important factor which needs consideration 10. Improve the internalization rate could enhance the antitumor efficacy in vivo. Cell penetrating peptide, which has been used to improve the internalization of biomacromolecules, have been proved that could increase the biofunction of integrated moiety 11. In our previous study, Ru Q et al showed that the integration of an arginine-rich cell penetrating peptide (Arg)9 with lidamycin could enhance the antitumor efficacy against glioma in vitro and in vivo 12. Based on the fusion protein dFv-LDP-AE 8, which consisted of two tandem anti-gelatianses scFv and an enediyne antibiotic lidamycin (LDM), an arginine penetrating peptide (Arg)9 was inserted into the framework of dFv-LDP-AE to enhance its efficacy of penetration into cell and named as dFv-R-LDP-AE (Fig. 1). The antitumor efficacies of dFv-LDP-AE and dFv-R-LDP-AE on pancreatic cancer were evaluated in vitro and in vivo.
Figure 1.
Diagram of gene fragments encoded for the LDP, dFv-LDP and dFv-R-LDP fusion proteins. The gene sequence of (Arg)9 was inserted between dFv and ldp. A linker of (G4S)2 was placed between dfv and (Arg)9, and a linker of G4S was placed between (Arg)9 and ldp gene.
Material and Methods
Ethics statement
The female athymic nude mice (Balb/c nu/nu, 6-8 weeks) were purchased from Beijing Vitalriver Experimental Animal Technology Co. Ltd., and allowed to acclimatize in the institutional animal house for >5 days before use. The animal experiments were approved by the Ethics Committee of The First Affiliated Hospital of Xinxiang Medical University, and carried out in accordance with a protocol approved by our hospital animal care and use committee and in compliance with institutional guidelines.
Cell culture
The human pancreatic cancer cell line SW1990, PANC-1 were routinely grown in RPMI-1640 (GIBCO) supplemented with 10% fetal bovine serum (GIBCO), penicillin-streptomycin (1%), and 2 mM L -glutamine.
Construction of the (Arg)9 contained pET-30a(+)/dFv-LDP expression vector
The diagram of plasmid dFv-LDP and dFv-R-LDP was described in Fig.1. The vector pET-30a(+)/dfv-ldp carried the gene for the anti-gelatinases scFv and the lidamycin apoprotein in the format a tandem scFv with LDP gene was constructed as described previously 8. DNA fragment encoding for (Arg)9-LDP was amplified by polymerase chain reaction (PCR) from the plasmid pET-(Arg)9-ldp, which described previously 12, using following primers: the forward primer 5'-CCCAAGCTTCGTCGTCGCCGCCGTCGTCGC-3' (the Hind III site is underlined) and the reverse primer 5'-GTTACTCGAGGCCGAAGGTCAGAGCCACGTG-3' (the XhoI site is underlined). The gene fragment was digested by endonucleases Hind III/XhoI, and mixed with a Hind III/XhoI-cleaved pET-30a(+)/ dfv-ldp vector and ligated overnight at 16 ℃ to construct vector pET-dfv-(Arg)9-ldp. The ligation products were transformed into E. coli DH5α and the plasmid sequencing confirmed the expected DNA sequences.
Preparation of dFv-LDP and dFv-R-LDP fusion protein
The sequence-verified plasmid pET-30a(+)/ dfv-r-ldp was transformed into expression strain E. coli BL21(DE3) to produce the recombinant protein. Expression, purification of dFv-R-LDP fusion protein was carried out according to the manufacturer's protocol (Novagen). The purified proteins were refolded in a way of stepwise dialysis as reported 8. The other format dFv-LDP fusion protein was produced similarly.
Affinity determination with antigen gelatinases and SW-1990
The antigen-binding activities of dFv-LDP or dFv-R-LDP with antigen gelatinases were detected by ELISA as described before 8. Briefly, serial dilutions of refolded dFv-LDP or dFv-R-LDP in 1% BSA-PBS were added into gelatinases pre-coated plates, incubated and washed. Then, the plate was incubated with anti-His-tag HRP-conjugate antibody and washed. 3.3 0 and 5.5 0 -tetramethylbenzidine was used as the chromogen for the color development, absorbance values at 450 nm were measured on microplate reader (Bio-Rad). For binding with PC SW-1990 cell, a flow-cytometry-based immunofluorescence assay was used to measure the binding affinity as described previously 13. The fusion protein dFv-LDP and dFv-R-LDP were FITC labeled for 16 h in a carbonate buffer solution [100 mmol/L NaHCO3, 10 mmol/L Na2CO3 (pH 9.0)] at 4℃. Labeled protein was separated from unbound FITC by using Sephadex G-25 column (GE Healthcare). Then the FITC-labeled dFv-LDP and dFv-R-LDP was incubated with SW-1990 cells (fixed with 70% ethanol for 24 h) in a 100 ml volume of buffer (PBS+2%FBS) for 2 h at room temperature. Following three washes with PBS buffer, cells were analyzed with FACS Calibur (BD company). The data were analyzed with Prism 5 software (GraphPad Software).
Internalization assay
To compare the internalization efficiency of each fusion protein to target cells, we used a fluorescence-activated cell sorting (FACS)-based assay. As described above, each FITC-labeled fusion protein, dFv-LDP or dFv-R-LDP was incubated with 0.5×106 live SW-1990 or PANC-1 cells in a 200 μL volume of FACS buffer (PBS+2% fetal bovine serum) for 1 h at room temperature. Then the 4% trypan blue was added to quench the extracellular FITC fluorescence. Following twice washes with 500 μL of FACS buffer, cells were analyzed with FACS Calibur (BD Company) to detect the intracellular FITC fluorescence 13.
Preparation of energized fusion protein
The energization of fusion protein dFv-R-LDP or dFv-LDP with the chromophore of lidamycin was carried out as described previously 8, 14. In short, the active enediyne chromophore (AE) of LDM was separated by using C4 column (GE Healthcare) with a 22% acetonitrile in 0.05% trifluoroactic acid mobile phase. The AE-containing solution was added to dFv-LDP/PBS (10 mmol/L; pH7.4), dFv-R-LDP/PBS, respectively, with the molecular ratio of 3:1, and was incubated at 4℃ for 12 h while rocking. Free AE was removed by using a Sephadex G-75 column (GE Healthcare) and assembled energized fusion proteins were confirmed by reverse-phase HPLC using a Vydac C4 300A column (Grace). Absorbance at 350 nm was measured.
MTT assay
The MTT assay was used for measuring in vitro cytotoxicity of energized fusion proteins as described previously 8. Cells were seeded in 96-well plates and incubated in 37℃ for 24 h and then exposed to different concentrations of dFv-R-LDP-AE, dFv-LDP-AE and dFv-LDP for 24 h. MTT (Sigma) solution (5 mg/mL, 20 μL) was added to each well and incubated for another 4 h at 37℃. The supernatant was removed and 150 μL DMSO were added to each well. The absorbance at 570 nm was measured using an ELISA reader (Thermo Fisher). Survival ratio was calculated according to the following formula: Survival ratio = (Atest -Ablank )/(Acontrol -Ablank )×100%. Experiment was performed in triple technical replicates (each with 3 biological replicates) and averaged.
FITC-Annexin V/PI apoptosis assay
Pancreatic cancer cell SW-1990 and PANC-1 were treated with dFv-LDP (2 nM), dFv-LDP-AE (0.001 nM) and dFv-R-LDP-AE (0.001 nM) for 24 h, and then the cells were collected and resuspended in 200 µl binding buffer. After that 10 µl FITC-labeled enhanced Annexin V and 100 ng propidium iodide were added. Upon incubation in the dark (15 min, room temperature), the samples were diluted with 500 µl binding buffer. Flow cytometry was carried out on a FACS Calibur (BD Company) and data were processed by its software.
Western blot analysis
For western blot, the overnight cultured Pancreatic cancer cell SW-1990 and PANC-1 were treated with serial diluted dFv-R-LDP-AE fusion protein (0.001 nM, 0.01 nM and 0.1 nM) for 24 h, and then the cells were collected and lysed for 30 min in radioimmunoprecipitation assay (RIPA) buffer at 4℃, which contained several protease inhibitors (e.g. 1 µg/mL aprotinin, 10 µg/mL leupeptin, 1 mmol/L phenylmethylsulfonyl fluoride, 2 mmol/L NaVO4 and 50 mmol/L NaF). Protein concentration was determined using bicinchoinic acid kit (Pierce Biochemicals), and 50 µg of each total protein were loaded on 10% SDS-PAGE and then electroblotted onto PVDF membranes (Millipore). The membranes were incubated with 5% BSA for 2 h at room temperature before incubation with the primary antibodies at 4℃ for overnight (diluted 1:1000 with TBST buffer, Cell Signaling Technology). Then the membranes were incubated with the secondary HRP-conjugated antibodies (1:5000 dilution; Cell Signaling Technology) for 2 h after washing 3 times with TBST buffer. The specific bands were visualized with the chemiluminescent HRP substrate kit (Millpore) and captured by the Amersham Imager 600 imaging system (GE health, USA).
In vivo antitumor experiments
The mouse SW1990 animal experiment was performed with 7-week-old female Balb/c mice. SW1990 cells suspended in saline were inoculated subcutaneously (Day 0) at the right axilla of the mouse with 2.0×106 cells/0.2 ml/mouse. At day 7, the tumors transplanted reached about 100 mg in size, the mice were randomized into treatment groups (n = 7 per group). Then the treatments were started (intravenously injection twice at day 7 after tumor implantation), the nude mice were injected in the tail vein with dFv-LDP-AE (0.25 mg/kg), dFv-R-LDP-AE (0.25 mg/kg) and dFv-LDP (5 mg/kg), respectively. Control nude mice were injected with saline. Tumor growth was measured with a caliper, and tumor volumes were calculated. The experiment was terminated at day 28, and the tumors were excised and weighed. The mean tumor weight was calculated and the results were expressed as the mean ± standard deviation. The tumor inhibition rate was calculated by: 1 - Tumor weight (treated) / tumor weight (control) × 100%.
Optical imaging
Optical imaging was performed with an IVIS-Imaging System (Xenogen). For establishment of xenografted and orthotopic transplantation human pancreatic tumor model was described previously 8, 15. Images and measurements of fluorescence signals were acquired and analyzed using Living Image-software (Xenogen). Ten to fifteen minutes prior to in vivo imaging, animals (n=3) received the FITC-labeled dFv-R-LDP at 10 mg/kg by tail vein injection and were anesthetized using 1-3% isoflurane. Animals were placed onto the warmed stage inside the camera box and received continuous exposure to 1-2% isoflurane to sustain sedation during imaging. The imaging time was 20 s and two to three mice were imaged at a time. The light emitted from the FITC was detected in vivo by the IVIS-Imaging System, then digitized and electronically displayed as a pseudocolor overlay onto a gray scale animal image.
Statistical analysis
Results of quantitative data in this study were presented as the mean ± SD. Unpaired two-tailed t-test was used to analyze the differences between groups, and P-values < 0.05 were considered statistically significant.
Results
Plasmid construction and protein preparation
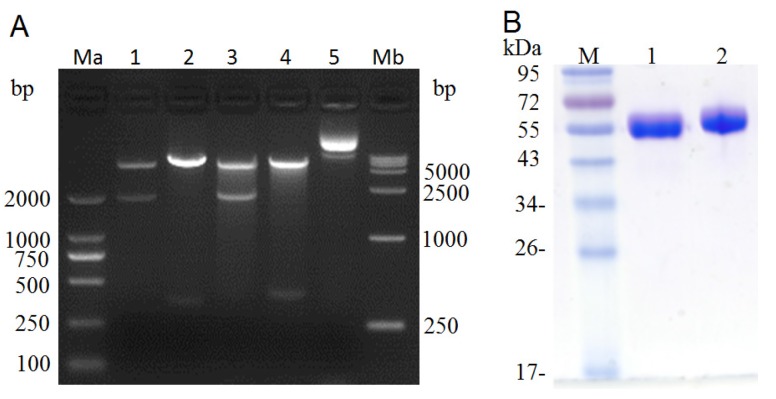
After PCR amplification, the (Arg)9 contained ldp gene was digested with Hind III / Xho I and then ligated with the Hind III / Xho I digested plasmid pET-dfv-ldp, which would replace the ldp partition in plasmid pET30(a+)/dfv-ldp. As shown in Fig.2A, when digested with Nde I/Xho I or Hind III/Xho I, it could find that the (Arg)9 contained plasmid pET-dfv-r-ldp displayed a little delayed migration in the agarose gel electrophoresis compared to that of pET-dfv-ldp. In lane 3 and 4 (Nde I/Xho I digested), the cleaved band (dfv-r-ldp) was migrated a little slowly compared to the lane 1 (the cleaved band dfv-ldp), because of the adding of (Arg)9 oligonucleotides. Similarly for the same reason, the cleaved band (r-ldp) in lane 4 (Hind III / Xho I digested) was migrated slowly compared to the rate of migration in lane 4 (ldp gene, Hind III / Xho I digested), which meant that the successfully insertion of (Arg)9 in the middle of dfv-ldp. The confirmed plasmid pET-dfv-r-ldp was transformed to the E.coli BL21(DE3), the protein expression was induced by adding of IPTG and purified by Ni2+ affinity chromatography. The graph of SDS-PAGE in Fig.2B showed that the protein dFv-R-LDP and dFv-LDP were well prepared.
Figure 2.
Digestion of plasmid dFv-LDP, dFv-R-LDP and its protein expression in E.coli. A: agarose gel electrophoresis of digested plasmid dFv-LDP and dFv-R-LDP. Lane 1 and 3, plasmid dFv-LDP and dFv-R-LDP were digested with Nde I/Xho I, respectively; Lane 2 and 4, plasmid dFv-LDP and dFv-R-LDP were digested with Hind III/Xho I, respectively; Lane 5, the plasmid pET30 a(+); Ma and Mb were the DNA marker DL2000 and DL15000, respectively. B: the SDS-PAGE analysis of purified protein dFv-LDP (lane 1) and dFv-R-LDP (lane 2), the molecule weight was about 65 kDa.
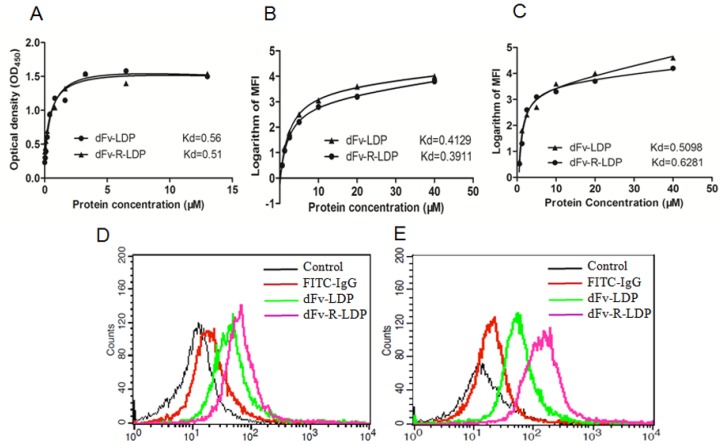
Affinity determination
The ELISA assay was adopted to detect the binding capability of dFv-LDP and dFv-R-LDP with the antigen gelatinases. As shown in Fig.3A, the binding of dFv-LDP and dFv-R-LDP with the gelatinases was similar, the affinity constants for dFv-LDP and dFv-R-LDP were 0.56 M and 0.51 M, respectively, which suggested that the adding of (Arg)9 did not have much influence on the binding capability with gelatinases. To further observe the binding capability, the FACS was also used to compare the affinity by monitoring the average fluorescence intensity (MFI), as shown in Fig.3B and Fig.3C, the binding capability of dFv-LDP and dFv-R-LDP with the pre-fixed SW1990 and PANC-1 cell did not have significant differences, the Kds of dFv-LDP and dFv-R-LDP with SW-1990 were 0.4129 M and 0.3911 M, respectively. And the Kds of dFv-LDP and dFv-R-LDP with PANC-1 were 0.5098 M and 0.6281 M, respectively.
Figure 3.
Characteristics of fusion protein dFv-LDP and dFv-R-LDP on affinity and penetration. A: Affinity determination of fusion protein dFv-LDP and dFv-R-LDP with antigen geltainases using ELISA assay; B and C: Affinity determination of fusion protein dFv-LDP and dFv-R-LDP with pancreatic cancer cell SW-1990 (B) and PANC-1 (C) using FACS; D and E: The penetration efficiency analysis of FITC-labeled fusion protein dFv-LDP and dFv-R-LDP using FACS in SW-1990 (D) and PANC-1 (E) cells, the concentration of FITC-labeled dFv-LDP and dFv-R-LDP used were 0.5 mM and the incubation time with SW-1990 and PANC-1 cells was 1 h.
Penetration efficacy
To investigate the (Arg)9 integration in fusion protein dFv-LDP whether improve the penetration efficacy or not, the FACS was used to analyze the possibility. As shown in Fig.3, FITC-labeled dFv-LDP displayed limited internalization compared to that of dFv-R-LDP in the time point of 1 h in SW-1990 (Fig.3D) and PANC-1 (Fig.3E) cells. In contrast, the (Arg)9 contained dFv-R-LDP showed apparent improvement in the penetration efficacy.
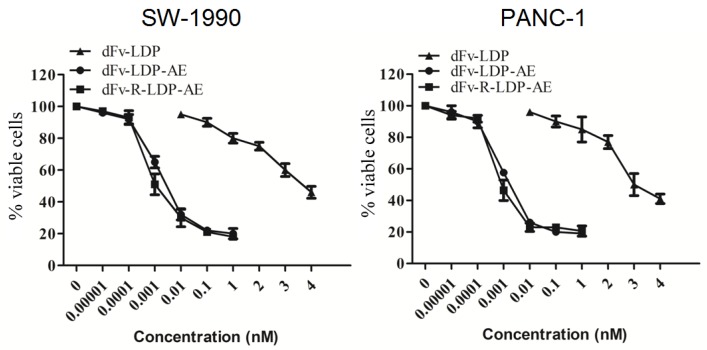
MTT assay
To energize the fusion protein dFv-R-LDP or dFv-LDP, the chromophore of lidamycin was energized with them to generate the fusion protein dFv-R-LDP-AE or dFv-LDP-AE. After that, the energized fusion protein dFv-R-LDP-AE and dFv-LDP-AE showed potent cytotoxicity against the pancreatic cancer SW1990 and PANC-1, as shown in Fig.4, the IC50 of dFv-LDP, dFv-LDP-AE and dFv-R-LDP-AE to SW1990 were 3.81×10-6, 4.38×10-12 and 1.37×10-12 M, respectively. And the IC50 of dFv-LDP, dFv-LDP-AE and dFv-R-LDP-AE to PANC-1 were 2.65×10-6, 3.15×10-12 and 8.37×10-13 M, respectively. Compared to that of dFv-LDP-AE, there was about 3~4 folds improvement in the cytotoxicity of dFv-R-LDP-AE to SW-1990 and PANC-1 cells in vitro as based on the MTT assay.
Figure 4.
Cytotoxicities of energized fusion protein dFv-LDP-AE and dFv-R-LDP-AE on SW-1990 (A) and PANC-1 (B) in vitro using MTT assay.
Apoptosis assay
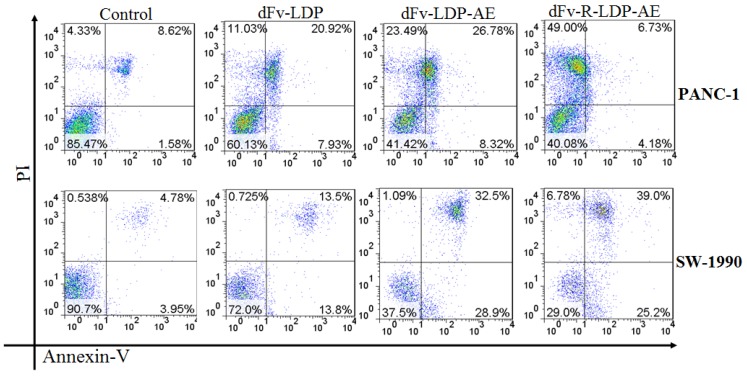
To compare the apoptosis induction capability of energized dFv-LDP-AE and dFv-R-LDP-AE, the Annexin V/PI double stained method was used. As shown in Fig.5, both of dFv-LDP-AE and dFv-R-LDP-AE showed robust apoptosis induction in PANC-1 and SW-1990 cell lines at concentration of 0.001 nM. In contrast, the dFv-R-LDP-AE displayed enhanced apoptosis induction compared to that of dFv-LDP-AE in SW-1990 cell, which might be caused by the improvement of the penetration efficiency of the energized fusion protein. Moreover, it was found that there were more necrotic cells rather than apoptotic cells in PANC-1 cells treated with dFv-R-LDP-AE and partially for PANC-1 treated with dFv-LDP-AE. The inconsistent tendency of PANC-1 and SW-1990 when treated with dFv-R-LDP-AE or dFv-LDP-AE might led by the difference of cell lines between PANC-1 and SW-1990, and which indicated that PANC-1 was more sensitive to the treatment.
Figure 5.
Apoptosis induced activities of energized fusion protein dFv-LDP-AE and dFv-R-LDP-AE on pancreatic cancer SW-1990 and PANC-1 cell using Annexin-V/PI assay. The concentration of dFv-LDP, dFv-LDP-AE and dFv-R-LDP-AE used were 2 nM, 0.001 nM and 0.001 nM, respectively.
Animal study
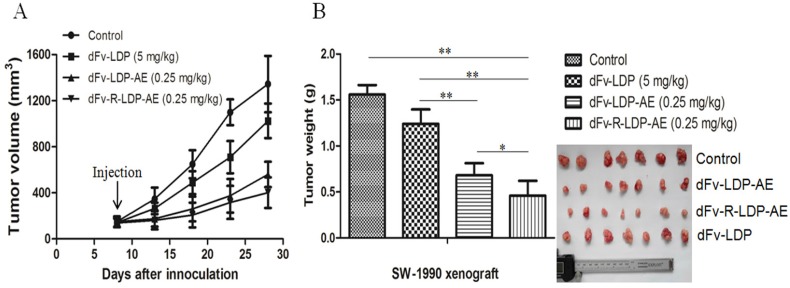
The xenografted SW-1990 pancreatic cancer was established in Balb/c (nu/nu) to evaluate the antitumor efficacy of dFv-LDP-AE and dFv-R-LDP-AE. As shown in Fig.6A and B, the fusion protein dFv-LDP showed certain antitumor efficacy with the tumor inhibition ratio of 20.42%, the energized fusion protein dFv-LDP-AE and dFv-R-LDP-AE showed the tumor growth inhibition rate of 56.31% (P<0.01, compared to dFv-LDP) and 74.2% (P<0.05, compared to dFv-LDP-AE), respectively, and body weight loss of the nude mice in whole experiment were less than 10% (data not shown).
Figure 6.
Antitumor activities of energized fusion protein dFv-LDP-AE and dFv-R-LDP-AE in SW-1990 xenografted mice. A was the tumor growth curve, the injection time was at day 7 and just for once. B was the net weight of excised tumor.
Western blot analysis
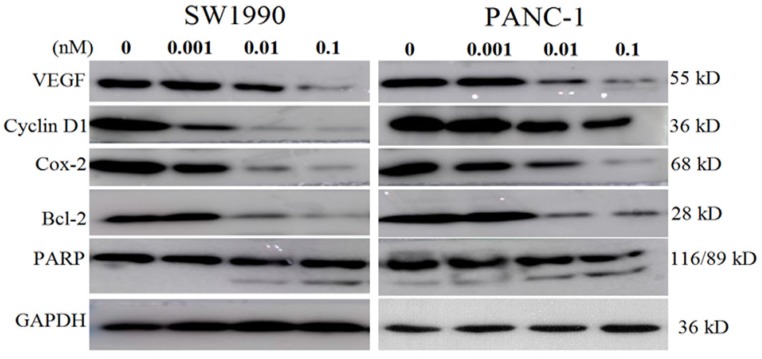
As the energized fusion protein dFv-R-LDP-AE demonstrated better antitumor efficacy, therefore the molecular mechanism underlied was performed to shed light on its antitumor efficacy. As shown in Fig.7, in pancreatic cancer cell line SW1990 and PANC-1, the treatment of dFv-R-LDP-AE could decrease the expression of VEGF, Cyclin D1, Cox-2, Bcl-2, and increase the cleavage of PARP in a dose dependent manner.
Figure 7.
Effects of energized fusion protein dFv-R-LDP-AE on certain signal pathway in vitro. Expression of apoptosis related molecules (e.g. PARP, Bcl-2) and VEGF, Cyclin D1, Cox-2 were determined by Western blot analysis, GAPDH was served as a loading control.
Optical imaging
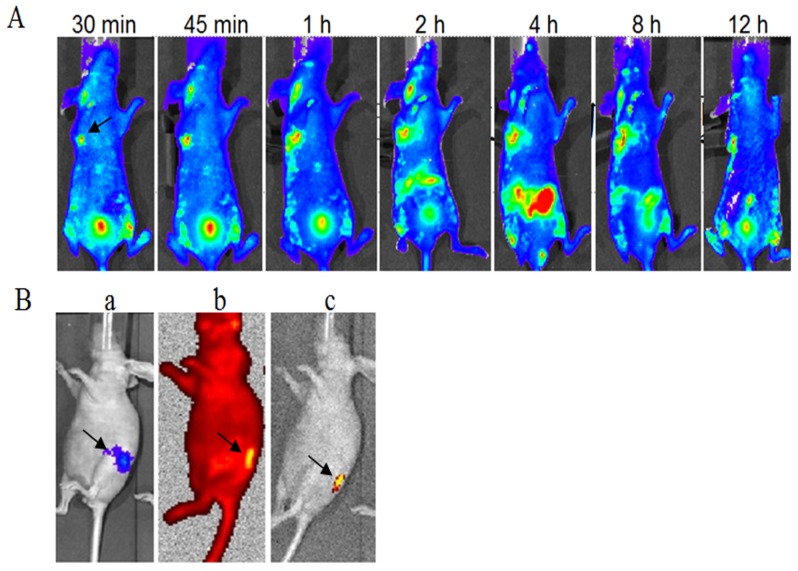
The FITC-labeled fusion protein dFv-R-LDP (0.5 mg/per mouse) was injected via the tail veil in SW1990 transplanted Balb/c nude mice to investigate the tumor targeting capability. As shown in Fig.8A, it could observed that the fluorescence was gathered at the transplanted tumor site at less than 30 min after the fusion protein injection, the intensity was increased with the time and reached the maximum at about 4 h. At the same time, the fluorescence in the abdomen of the mice was also increased at 4 h, which suggested the elimination of fusion protein. The FITC fluorescence at 12 h at the tumor loci was still apparent, which demonstrated the good targeting capability of fusion protein dFv-R-LDP to the xenografted SW1990 pancreatic cancer.
Figure 8.
Optical imaging of FITC-labeled fusion protein dFv-R-LDP in xenografted SW-1990 tumor and orthotopic Capan-2 Luc+ pancreatic cancer transplanted mice in vivo. A: targeting capability of FITC-labeled dFv-R-LDP in xenografted SW-1990 mice, the arrow indicated the location of tumor; B: targeting capability of FITC labeled dFv-R-LDP in orthotopic Capan-2 mice, the pancreatic cancer cell Capan-2 was Luc+ and orthotopic transplanted in the mice. a indicated the location of Capan-2 tumor, luciferase substrate D-luciferin (150 mg/kg) was injected intraperitoneally, and the animals were placed onto the warmed stage inside the camera box (IVIS-Imaging System, Xenogen) to locate the tumor; b: FITC-labeled dFv-R-LDP was injected via tail vein, and the graph was gotten 1 hour later after injection under the high-sensitive red-filter xenogeny system; c: the graph obtained from b, which removed the red-filter.
To further prove the tumor targeting capability of dFv-R-LDP to pancreatic cancer, the mice of orthotopic transplanted PC Capan-2 with luciferase transfected was generated, as shown in Fig.8B, the orthotopic mice model with Capan-2Luc+ was successfully established after the luciferase substrate D-luciferin (150 mg/kg) injection via intraperitoneal approach. Two hours later, the luciferase signal was disappear and then the FITC-labeled fusion protein dFv-R-LDP was injected via tail veil, the graph captured showed that the fusion protein could prominent accumulate at the tumor foci under the high resolution red optical filter or the grey background after the withdrawal of the red filter.
Discussion
Pancreatic cancer is one of the most dangerous malignancies to human health, and until now there is no very effective therapeutic method for treatment. Gelatinases (MMP-2/9) is a tumor associated antigen and highly related with the tumor progression 6, 7. It is reported the serum level of MMP-9 was a significant independent prognostic factor for the pancreatic cancer patients' survival 16, and the co-expression of MMP-9 and Tenascin-C in pancreatic cancer tissues was significantly associated with the progression and prognosis 17. Therefore, targeting therapy against gelatianses might present a potential approach for the treatment of pancreatic cancer. In our previous study, the anti-gelatinases scFv with lidamycin fusion protein dFv-LDP-AE demonstrated potent antitumor efficacy in lung cancer and liver cancer 8, 9 , it is known that the internalization property for an antibody or its conjugate is very important to maximize it biofunction, so in order to further improve the antitumor efficacy, in this study, an (Arg)9 penetrating peptide was integrated into the dFv-LDP to generate the dFv-R-LDP fusion protein (Fig.1), after that, the antitumor efficacy of two energized fusion protein dFv-LDP-AE and dFv-R-LDP-AE against pancreatic cancer were evaluated.
As shown in Fig.3, the integration of (Arg)9 in the dFv-LDP did not impair the binding capability with the antigen gelatianse and PC, whereas it could enhance the internalization rate of the fusion protein efficiently, the improvement of penetration was very apparent. As the AE of lidamycin was not very stable and could be self-decomposition via arylation 18, which was also confirmed via our HPLC analysis (Fig. S1 A and B), therefore, the fast entering of the cell and causing the DNA damage could maximize the antitumor function of AE or its conjugates. Therefore, the energized fusion protein dFv-R-LDP-AE showed enhanced cytotoxicity compared to that of dFv-LDP-AE in the MTT assay, which might cause by the improved penetration rate of the fusion protein. Also, the improvement of cytotoxicity demonstrated in the apoptotic induction, it was shown that the dFv-R-LDP-AE was more potent than the dFv-LDP-AE in the induction of apoptosis in SW-1990 and PANC-1. With the adding of (Arg)9 in the format of dFv-LDP, the internalization rate of fusion protein were improved, and therefore the enhancement of the antitumor efficacy in vivo, as shown in Fig.6 A and B, the antitumor efficacy of dFv-R-LDP-AE was better compared to that of dFv-LDP-AE (P < 0.05).
For the mechanism under the antitumor efficacy of dFv-R-LDP-AE, the results of Western blot showed that the deregulated expression of VEGF, Cyclin D1, Cox-2 and Bcl-2, which might reflected in the inhibition of angiogenesis, cell cycle, inflammation and the acceleration of cell apoptosis. At the same time, the increased cleavage of PARP meant the augment of apoptosis which were induced by the dFv-R-LDP-AE in a dose-dependent manner. The optical imaging of the animal study using the FITC-labeled dFv-R-LDP demonstrated that the fusion protein possessed well pancreatic cancer targeting capability in the xenografted and orthotopic mice model, which suggested that the gelatinases could be a possible target for antibody targeting therapy in pancreatic cancer.
In sum, the results in this study demonstrated that antigelatinase scFv fused with the lidamycin had very good tumor inhibition efficacy in vitro and in vivo. As the fast internalization of lidamycin-contained fusion protein into the cancer cells was very important, the adding of a cell penetrating peptide (Arg)9 in the skeleton of which could further improve the antitumor function in vitro and in vivo. Therefore, the energized fusion protein dFv-R-LDP-AE might have potential application in the treatment of pancreatic cancer.
Supplementary Material
Supplementary figure S1.
Acknowledgments
This study was supported by the Natural Science Foundation of China (grant No. 81201765), Key Scientific Research Projects for Higher Education of Henan Province (grant No. 17A310022; 15A320063), the Young Backbone Teachers Fellowship in Henan Province (2016GGJS-105) and the young scientist project in the First Affiliated Hospital of Xinxiang Medical University (QN-2017-A010).
References
- 1.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 2.Castellanos E, Berlin J, Cardin DB. Current treatment options for pancreatic carcinoma. Curr Oncol Rep. 2011;13:195–205. doi: 10.1007/s11912-011-0164-1. [DOI] [PubMed] [Google Scholar]
- 3.Shao RG, Zhen YS. Enediyne anticancer antibiotic lidamycin: chemistry, biology and pharmacology. Anticancer Agents Med Chem. 2008;8:123–131. doi: 10.2174/187152008783497055. [DOI] [PubMed] [Google Scholar]
- 4.Guo XF, Zhu XF, Gao HY, Zhong GS, Li L, Deng BG. et al. A bispecific enediyne-energized fusion protein targeting both epidermal growth factor receptor and insulin-like growth factor 1 receptor showing enhanced antitumor efficacy against non-small cell lung cancer. Oncotarget. 2017;8:27286–27299. doi: 10.18632/oncotarget.15933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao R, Li L, Shang B, Zhao C, Sheng W, Li D. A gelatinases-targeting scFv-based fusion protein shows enhanced antitumor activity with endostar against hepatoma. Basic Clin Pharmacol Toxicol. 2015;117:105–116. doi: 10.1111/bcpt.12379. [DOI] [PubMed] [Google Scholar]
- 6.Jakubowska K, Pryczynicz A, Januszewska J. et al. Expression of matrix metalloproteinases 2, 7, and 9 in carcinogenesis of pancreatic ductal adenocarcinoma. Dis Markers. 2016;2016:9895721.. doi: 10.1155/2016/9895721. doi: 10.1155/2016/9895721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiang T, Xia X, Yan W. Expression of matrix metalloproteinases-2/-9 is associated with microvessel density in pancreatic cancer. Am J Ther. 2017;24(e):431–434. doi: 10.1097/MJT.0000000000000424. [DOI] [PubMed] [Google Scholar]
- 8.Zhong G, Zhang S, Li Y, Liu X, Gao R, Miao Q, Zhen Y. A tandem scFv-based fusion protein and its enediyne-energized analogue show intensified therapeutic efficacy against lung carcinoma xenograft in athymic mice. Cancer Lett. 2010;295:124–133. doi: 10.1016/j.canlet.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Zhong GS, Wu MN, Guo XF, Zhang SH, Miao QF, Zhen YS. Antitumor activities of dFv-LDP-AE: an enediyne-energized fusion protein targeting tumor-associated antigen gelatinases. Oncol Rep. 2012;28:1193–1199. doi: 10.3892/or.2012.1910. [DOI] [PubMed] [Google Scholar]
- 10.Xu S. Internalization, trafficking, intracellular processing and actions of antibody-drug conjugates. Pharm Res. 2015;32:3577–83. doi: 10.1007/s11095-015-1729-8. [DOI] [PubMed] [Google Scholar]
- 11.Skotland T, lversen TG, Torgersen ML, Sandvig K. Cell-penetrating peptides: possibilities and challenges for drug delivery in vitro and in vivo. Molecules. 2015;20:13313–13323. doi: 10.3390/molecules200713313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ru Q, Shang BY, Miao QF, Li L, Wu SY, Gao RJ, Zhen YS. A cell penetrating peptide-integrated and enediyne-energized fusion protein shows potent antitumor activity. Eur J Pharm Sci. 2012;47:781–9. doi: 10.1016/j.ejps.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Guo XF, Zhu XF, Shang Y, Zhang SH, Zhen YS. A bispecific enediyne-energized fusion protein containing ligand-based and antibody-based oligopeptides against epidermal growth factor receptor and human epidermal growth factor receptor 2 shows potent antitumor activity. Clin Cancer Res. 2010;16:2085–2094. doi: 10.1158/1078-0432.CCR-09-2699. [DOI] [PubMed] [Google Scholar]
- 14.Zhong GS, Guo XF, Zhang SH, Zhen YS. Optimization of the assembly efficiency for lidamycin chromophore bound to its apoprotein: a case study using orthogonal array. Biomed Environ Sci. 2011;24:602–607. doi: 10.3967/0895-3988.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, Zhang H, He H. et al. In vivo real-time imaging of gemcitabine-leaded growth inhibition in the orthotopic transplantation model of human pancreatic tumor. Acta Pharmaceutica Sinica B. 2011;1:220–225. [Google Scholar]
- 16.Mroczko B, Lukaszewicz-Zajac M, Wereszczynska-Siemiakowska U, Groblewska M, Gryko M, Kedra B, Jurkowska G, Szmitkowski M. Clinical significance of the measurements of serum matrix metalloproteinase-9 and its inhibitor (tissue inhibitor of metalloproteinase-1) in patients with pancreatic cancer: metalloproteinase-9 as an independent prognostic factor. Pancreas. 2009;38:613–618. doi: 10.1097/MPA.0b013e3181a488a0. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y, Li Z, Jiang P, Wu G, Chen K, Zhang X, Li X. The co-expression of MMP-9 and Tenascin-C is significantly associated with the progression and prognosis of pancreatic cancer. Diagn Pathol. 2015;10:211. doi: 10.1186/s13000-015-0445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoue M, Usuki T, Lee N, Hirama M, Tanaka T, Hosoi F, Ohie S, Otani T. Antitumor enediyne chromoprotein C-1027: mechanistic investigation of the chromophore-mediated self-decomposition pathway. J Am Chem Soc. 2006;128:7896–7903. doi: 10.1021/ja060724w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure S1.