Figure 4.
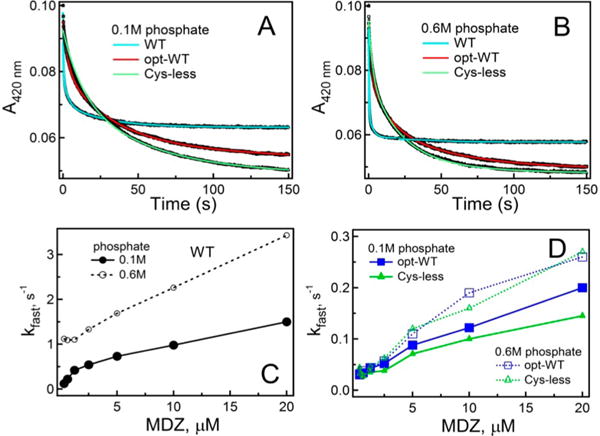
Kinetics of MDZ binding to WT, opt-WT, and Cys-less CYP3A4. (A and B) Kinetic traces obtained after mixing 2 μM proteins and 40 μM MDZ in 0.1 and 0.6 M phosphate buffer (pH 7.4), respectively. Kinetics were biphasic for the entire MDZ concentration range. Solid cyan, red, and green lines are fitting curves. (C) Dependence of kfast on MDZ concentration derived for WT CYP3A4. (D) Plots of kfast vs MDZ concentration for opt-WT and Cys-less CYP3A4. The kfast and kslow values measured at the maximal MDZ concentration are listed in Table 1.
