Figure 7.
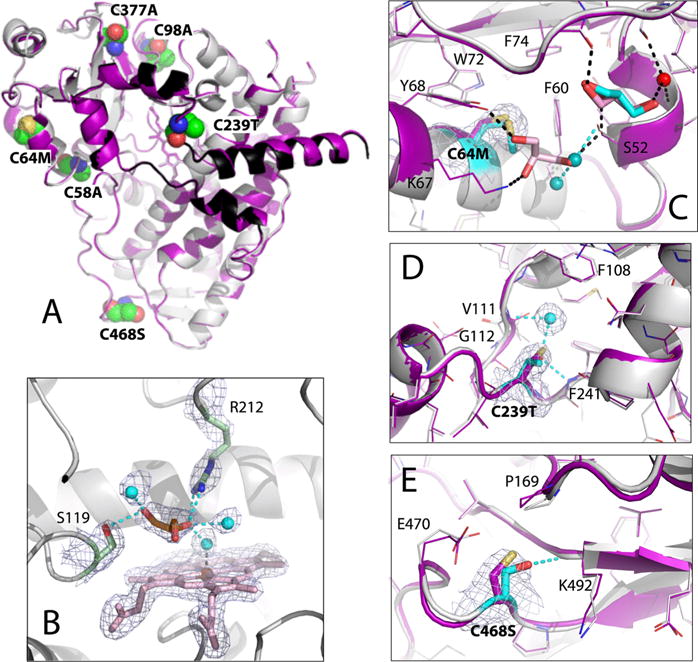
Crystal structure of Cys-less CYP3A4. (A) Superposition of Cys-less (gray and black) and opt-WT CYP3A4 (purple). The mutated residues are in CPK representation and labeled. (B) View of the active site where glycerol (orange sticks) forms H-bonds with the Ser119 and Arg212 side groups and three water molecules (cyan spheres). The 2Fo – Fc electron density map contoured at 1σ is shown as gray mesh. (C–E) Structural changes at the C64M, C239T, and C468S mutational sites, respectively. The superimposed Cys-less and and opt-WT structures are rendered in gray/cyan and purple, respectively. In Cys-less CYP3A4, the newly recruited water molecules and glycerol are shown as cyan spheres and cyan sticks, respectively; cyan dotted lines are the newly formed H-bonds. Water, glycerol, and H-bonds observed in opt-WT CYP3A4 are represented in the same manner but colored red, pink, and black, respectively. The 2Fo – Fc electron density maps at the mutation sites are shown as gray mesh contoured at 1σ.
