Abstract
Dietary consumption of polyphenol-rich fruits, such as grapes, may reduce inflammation and potentially prevent diseases linked to inflammation. Here, we used a genetically engineered murine model to measure Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-κB) activity and pro-inflammatory cytokine secretion to test the hypothesis that oral consumption of whole grape formulation reduces inflammatory signaling in the body. NF-κB luciferase reporter mice were divided into two groups, one which was fed an experimental diet formulated with 4% (w/w) whole grape powder (WGP) or another which was fed a control diet formulated with 3.6% glucose/fructose (w/w) combination. Simulated inflammation was induced in the mice by intraperitoneal injection of lipopolysaccharide (LPS). In vivo imaging was used to determine the effect of each diet on NF-κB activity. We found that there were no significant differences in weight gain between the WGP and control diet groups. However, there was a statistically significant (p<0.0001) difference in the progression of basal levels of NF-κB signaling between mice fed on control or WGP diet. There were no significant differences in NF-κB reporter indices between WGP- and control-diet groups after either acute or repeated inflammatory challenge. However, terminal blood collection revealed significantly (p<0.01) lower serum concentrations of the inflammatory cytokines Interleukin-6 (IL-6) and Tumor Necrosis Factor alpha (TNFα) only among WGP diet mice subjected to acute inflammatory challenge. Overall, these data suggest that while diets supplemented with WGP may suppress steady-state low levels of inflammatory signaling, such a supplementation may not alleviate exogenously induced massive NF-κB activation.
Keywords: Whole grape powder, inflammation, NF-kB, diet, in vivo, mouse
1. Introduction
Chronic, persistent inflammation is known to be associated with most chronic diseases such as diabetes, kidney disease, asthma, arthritis, cardiovascular diseases, Alzheimer’s disease and cancer [1–4]. These chronic conditions are the leading causes of human and companion animal morbidity and mortality both within the United States (U.S.) and worldwide [2]. The human health and economic consequences of these illnesses are immense. According to the U.S. Centers for Disease Control and Prevention (CDC), 50% of all adults in the U.S. suffer from at least one type of chronic illness, with approximately 75% of all deaths within the U.S. attributed to these chronic diseases [2, 5]. Though dietary and medical interventions may be able to reduce these numbers, with the continued rise in the obesity epidemic in the U.S., there is no indication that these diseases will be eradicated in the near future [6, 7]. Although many efforts have been placed on costly treatments of these chronic conditions, the investment in prevention of these conditions is still below the threshold for health outcome effectiveness. Recently, in recognition of the seriousness of the problem, research on the molecular process of inflammation and the development of preventive and therapeutic strategies has come to the forefront in biomedical research (NCI provocative questions, http://provocativequestions.nci.nih.gov).
One of the primary molecular drivers of inflammatory signaling in cells is the Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-κB), a dimeric protein which, in response to stimuli, translocates from the cytoplasm to the nucleus to initiate the expression of several genes which collectively promote multiple inflammation-associated pathologic processes [8–12]. Studies from our group and others have also shown the pathways and molecular regulation of NF-κB activation through various regulatory interactions [13–15]. In particular, canonical NF-κB signaling pathways induced by tumor necrosis factor alpha (TNF-α) and interleukin-1 (IL-1) play an important role in the pathogenesis of multiple chronic inflammatory diseases including inflammatory bowel disease (IBD), asthma, and chronic obstructive pulmonary disease (COPD) [12, 16]. Therefore, inhibition of NF-κB activity and/or the stimuli that induce NF-κB activity have become central themes for anti-inflammatory research [17–22]. Supporting evidence for the role of NF-κB driven inflammation in malignancies also comes from genetic models of diminished NF-κB signaling [8, 23]. As a result of its role in driving inflammation, the NF-κB pathway has also been targeted in therapeutic development and in various clinical trials [24–31].
Whole grape powder (WGP) contains several bioactive ingredients, including resveratrol, flavonoids, anthocyanins, cathechins, and other compounds. Several studies have documented that these ingredients contained in WGP possess anti-inflammatory and health promoting properties [32–41]. However, less is known about the activity of WGP as a composite entity that possesses a combinatorial activity of its ingredients. Therefore, studies using whole grape powder may help to elucidate and fully exploit the benefits from synergy among the individual bioactive components. This study was designed to determine the bioactivity of WGP in vivo by using a reporter mouse model for NF-κB-driven inflammatory signaling.
2. Materials and Methods
2.1. Experimental Animals and Groups
Five-week old female BALB/c-Tg(Rela-luc)31Xen (cat. no. 10499-F, n=40) reporter mice were purchased from Taconic Biosciences® (Hudson, NY) and housed in groups of 5 per cage in a 12 h light/12 h dark cycle, temperature-controlled room. After arrival, animals were allowed to acclimatize for 10 days before the start of experiments. At the end of the quarantine period the animals were randomly grouped into WGP (n=20) and control (n=20) diet groups. After 3 weeks on their assigned diets, 2 cages (10 animals) from each group were randomly assigned to either acute or repeat challenge group. Bodyweights of animals were recorded once a week and daily during the repeat challenge period. Animals were housed at the Tuskegee University Comparative Medicine Resource Center animal facility (Tuskegee Institute, AL) and all experiments described herein were reviewed and approved by the Tuskegee University Animal Care and Use Committee.
2.2. Whole Grape Powder (WGP)
Whole Grape Powder formulated from lyophilized red, green, blue-purple grapes was provided in aluminum bags by the California Table Grape Commission (Fresno, CA, USA). The bags were kept frozen at −40°C until use in feed formulation and during the entire study duration. The phytochemical and nutritional compositions of WGP used in this study are shown in Tables 1 and 2. The grape powder contains about 90% sugar in equal proportion of glucose and fructose.
Table 1.
Phytochemical analysis of the WGP powder used in the study*
| Compounds | Total | Individual |
|---|---|---|
|
| ||
| Catechins | mg/kg | |
|
| ||
| Catechin | 19.59 mg/kg +/− 1.06 | |
|
| ||
| Epicatechin | 8.77 mg/kg +/− .71 | |
|
| ||
| Anthocyanins | mg/kg | |
|
| ||
| Peonidin | 148.75 mg/kg +/− 8.19 | |
|
| ||
| Cyanidin | 20.1 mg/kg +/− 1.33 | |
|
| ||
| Malvidin | 127.77 mg/kg +/− 8.33 | |
|
| ||
| Flavonols | ||
|
| ||
| Kaempferol | 1.03 mg/kg +/− .16 | |
|
| ||
| Isorhamnetin | 1.06 mg/kg +/− .11 | |
|
| ||
| Quercetin | 14.44 +/− 1.20 | |
|
| ||
| Taxifolin | 1.87 mg/kg +/− .10 | |
| Stilbenes | ||
|
| ||
| Resveratrol | .85 mg/kg +/− .16 | |
|
| ||
| Total Polyphenols in gallic acid equivalents | 326 mg/100g | |
Data provided by the supplier.
Note: This analysis does not represent the complete phytochemical profile of grapes. Abbreviations: kg = kilogram, g = gram, mg = milligrams, mcg = micrograms, IU = international unit
Table 2.
Nutritional analysis of WGP used in this study*
| Nutrient | Amount (per 100 g powder) | Units |
|---|---|---|
| Calories | 371 | kcals |
| Total Fat, acid hydrolysis | 0.299 | g |
| Total Carbohydrate | 88.6 | g |
| Protein (N x 6.25) | 3.58 | g |
| Beta carotene | 0.127 | mg |
| Vitamin A from carotene | 212 | IU |
| Vitamin C | 2.7 | mg |
| Calcium | 50 | mg |
| Iron | 1.43 | mg |
| Sodium | 11.8 | mg |
| Potassium | 973 | mg |
| Thiamin HCl | 0.17 | mg |
| Folic Acid | 49.0 | mcg |
| Phosphorus | 104 | mg |
| Magnesium | 33.3 | mg |
| Zinc | 0.416 | mg |
| Copper | 0.450 | mg |
| Manganese | 0.379 | mg |
| Moisture | 4.52 | g |
| Ash | 3.02 | g |
Data provided by the supplier.
Abbreviations: g = gram, mg = milligrams, mcg = micrograms, IU = international units, kcals = kilocalories
2.3. Feed Formulation
Powdered rodent diet was purchased from (Teklad® T.2018M.15, Harlan Laboratories, Indianapolis, IN) and formulated in-house into mashed feed supplemented by addition of 4% WGP (experimental, w/w) or 3.6% sugar (control, 1:1 w/w mixture of glucose and fructose). The powdered feed was combined with fresh WGP or control supplement every day in the morning, made into paste using deionized water and then molded into 14 g balls which were placed daily on mice cage feeders. Five balls were placed in each cage daily for the entire duration of the study. Any remaining feed material was discarded before the fresh balls were placed.
2.4. Induction of Inflammatory Stimuli
To induce inflammation in the mice, we injected lipopolysaccharide (LPS, L3024, Sigma-Aldrich, Co., St. Louis, MO) intraperitoneally (i.p.). Acute inflammatory challenge was induced by one time administration of 0.5 mg/kg LPS. Repeat challenge inflammation was induced by administration of 0.25 mg/kg LPS every other day during the final week of the study. Luminescence images for both acute and repeat challenges were taken 4 hours after the LPS injections.
2.5. In vivo Imaging and Sera Collection
BALB/c-Tg(Rela-luc)31Xen mice are genetically engineered model mice carrying a reporter construct which expresses luciferase enzyme when NF-kB signaling is activated in the body. Subsequent injection of luciferin, a substrate for luciferase, results in a luminescent signal measured by in vivo imaging. Animals were administered luciferin substrate solution (XenoLight D-Luciferin, PerkinElmer, Santa Clara, CA) equivalent to 15 μg/kg of animal weight via i.p. injection. After 15 minutes, animals were placed in the imager in supine position and imaged two at a time using IVIS Lumina XR In Vivo Imaging System (PerkinElmer, Santa Clara, CA). In vivo luminescence images were taken before the diet provision, and then once a week for the remainder of the study period except for those animals on repeat challenge schedule, which were imaged on each of the challenge days. Luminescence measurements were collected and analyzed as average radiance (photon/sec/cm2/sr) for regions of interest drawn over the entire body excluding the head, and the abdominal/peritoneal region. Terminal sera were collected and saved for the analysis of TNFα and IL-6 (a model NF-κB transcriptional target gene) as inflammation marker cytokines.
2.6. Inflammatory Cytokine Analysis
Serum concentration of the inflammatory cytokines TNFα and IL-6 were determined using the Quantikine® ELISA kit for mouse TNF-alpha (Cat#MTA00B) and Quantikine® ELISA kit for mouse IL-6 (Cat#M6000B), both from R&D Systems (Minneapolis, MN), as recommended by the manufacturer. Samples were run in duplicates and results were averaged.
2.7. Statistical Analysis
All results are presented as means ± standard deviation of the means. Observed differences in bodyweight changes between WGP-fed experimental groups and their respective controls were analyzed using an unpaired two-tailed student’s T-test (α=0.05). Similarly, changes in baseline NF-κB luciferase reporter activity over the period in which the animals received experimental diet prior to inflammatory challenge were analyzed using paired two-tailed student’s T-test (α=0.05). Observed differences in NF-κB luciferase reporter activity and serum inflammatory cytokine concentrations following inflammatory challenges were determined using an unpaired two-tailed student’s T-test with Welch’s correction (α=0.05). All statistical procedures were performed using Graphpad Prism, version 6.0 (GraphPad Software, Inc., La Jolla, CA).
3. Results
3.1. Body weight changes
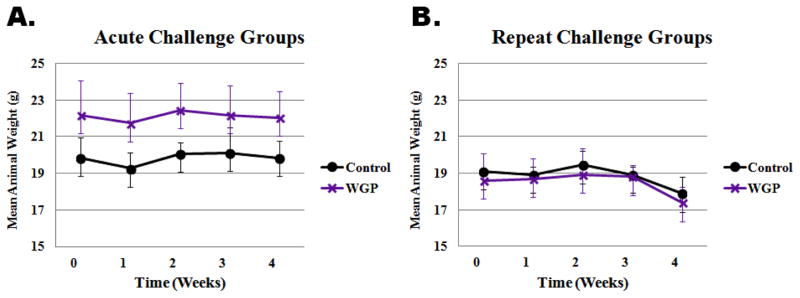
Weekly bodyweight measurements were taken to determine if the differential feeding of mice on WGP or control (fructose/glucose) diets would affect body weight gain. Analysis of the measurements for both control diet and WGP diet showed no difference in bodyweight gain during this study. Figure 1 shows the average bodyweight plots for the two groups. The results indicate that both groups followed similar trends under both acute (A) as well as repeat-challenge (B) experimental setups. Mice in acute challenge groups did not lose weight, nor was there any difference in trend between the control and WGP diet groups. However, both control and WGP diet mice in the repeat challenge groups lost body weight during the challenge week. The drop in bodyweight observed was not different between the control and WGP diet groups. Therefore, under these experimental conditions, WGP diet did not affect trends in mice body weight.
Figure 1.
Bodyweight trend of mice on control or whole grape powder (WGP) supplemented diet. Mice were randomly grouped into either acute challenge or repeat challenge group, each of which was further sub-grouped into control or WGP diet. Weekly bodyweight measurements were taken. Plots represent average weight of animals (n=10 per group) versus time in weeks. Panel A is shows data plot for animals in the acute challenge group, while panel B shows plot for the repeat challenge group. WGP supplementation for 3 weeks did not affect bodyweight trend.
3.2. Effect of WGP supplementation on changes in basal endogenous NF-κB reporter activity in mice
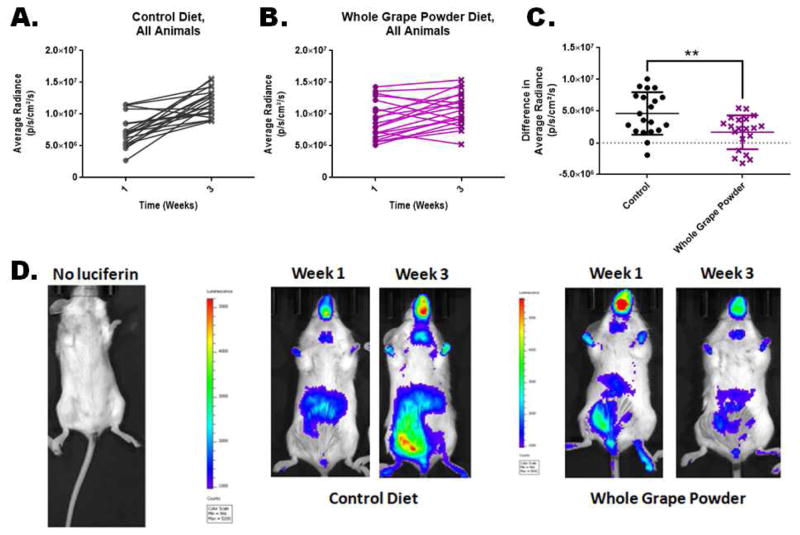
The experimental mice, which are immunocompetent, were housed under conventional husbandry. After the animals were housed, we identified each animal individually and weekly measured the reporter activity by sequential in vivo imaging for three weeks. The mean full-body luminescence values from individual mice during the 3 weeks of feeding period were analyzed. We compared the luminescence data from the control diet and WGP diet groups for change in basal reporter activity in unchallenged animals. Paired-sample plots for basal endogenous NF-κB reporter activity in mice fed control (Figure 2A, n=20) or WGP (Figure 2B, n=20) diet for 3 weeks is shown. Mean values for changes in reporter activity (as the difference in radiance units between week 1 and week 3) over the same period of time are represented by the dot plot in Figure 2C. The data showed significant (p<0.0001) difference in the rate of change in reporter activity (plotted as difference in radiance units on Y-axis) between the two groups over the period of 3 weeks. The control diet groups showed a steep increase (slope) and greater difference (delta values) in radiance units within the 3 weeks period compared to the WGP group. Examples of luminescence images from a non-injected mouse and mice from either control diet group or WGP diet group are shown in Figure 2D. Therefore, the results suggest that WGP diet slows down the rate of steady state endogenous NF-κB activity in vivo.
Figure 2.
Effect of WGP supplementation on changes in basal NF-κB activity in reporter mice. Paired-sample plots for basal NF-κB reporter activity in mice fed control (Panel A, n=20) or WGP (Panel B, n=20) diet for 3 weeks. Changes in reporter activity over the 3 weeks for each of the animals in either control- or WGP-diet group are shown in panel C. Panel D shows luminescence images from mouse with no luciferin injection (left) and from a mouse in control diet group (at week 1 and week 3, middle panels), and from a mouse in WGP diet group (also at week 1 and week 3, right panels). The WGP group had significantly lower rate of increase in reporter activity over the 3 weeks period. ** statistically significant difference (p<0.0001).
3.3. Effect of WGP supplementation on changes in NF-κB reporter activity in mice subjected to acute inflammatory challenge
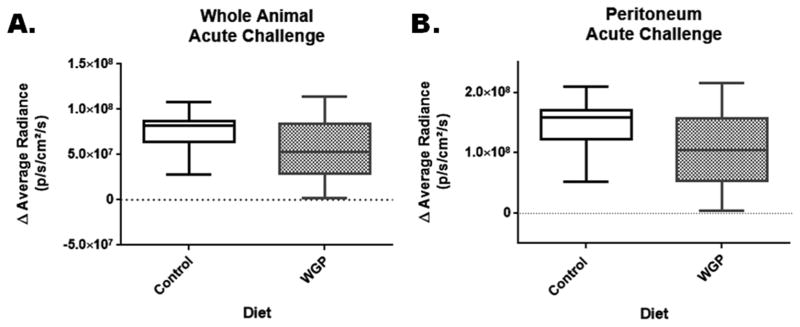
After the 3-week feeding period and after the basal steady state reporter activity measurements, animals in the acute challenge group (n=10 for control diet and n=10 for WGP diet) were injected i.p. with 0.5 mg/kg LPS and in vivo images were taken 4 hours later as post-challenge measurements. We used the pre-challenge week 3 (last time point) measurements as a baseline values to calculate the differences in reporter activity between pre- and post-acute challenge. Box plots in Figure 3A and 3B represent the differences in mean values for reporter activity in the full-body (panel A) and abdominal/peritoneal region (panel B), respectively, as measured in radiance units. Analysis of the results showed that there were no significant differences between the reporter activity measurements between the two diet groups.
Figure 3.
Effect of WGP supplementation on changes in NF-κB reporter activity in mice subjected to acute inflammatory challenge. After 3 weeks on diet, mice were subjected to an acute inflammatory challenge. Changes in reporter activity were plotted as the difference between the pre-challenge (week 3 readings) and post-acute challenge readings. Box plots represent the differences in reporter activity for the whole body (panel A) and abdominal/peritoneal (panel B) regions of interest. There was no significant difference between control and WGP groups.
3.4. Effect of WGP supplementation on changes in NF-κB reporter activity in mice subjected to repeat inflammatory challenge
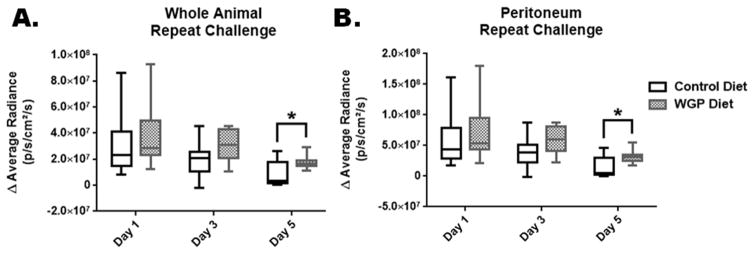
For repeat challenge, mice were injected i.p. with a lower dose of LPS (0.25 mg/kg) on day 1, day 3 and day 5. In vivo imaging was done on each day of injection 4 hours after the injections. Similarly to the acute challenge experiments, we computed differences in reporter activities between the pre-challenge week 3 and repeat challenge values. Box plots in Figure 4A and 4B represent the mean differences in reporter activity in the full body (panel A) and abdomen/peritoneum (panel B). Analysis of results from these experiments showed that there were no significant differences between control and WGP groups for days 1 & 3 (p>0.6). Significance was reached (p=0.03) for day 5 with higher readings for the WGP group, yet very close to the cut off value of α=0.05.
Figure 4.
Effect of WGP supplementation on changes in NF-κB reporter activity in mice subjected to repeat inflammatory challenge (on days 1, 3, and 5). Changes in reporter activity were plotted as the difference between the pre-challenge (week 3 readings) and post- challenge readings on each day of challenge. Box plots represent the differences in reporter activity for the whole body (panel A) and abdominal/peritoneal (panel B) regions of interest. There were no significant differences between control and WGP groups for days 1 & 3 (p>0.06). Significance was reached (*, p=0.03) for day 5.
3.5. Effect of WGP supplementation on serum levels of the cytokines IL-6 and TNFα
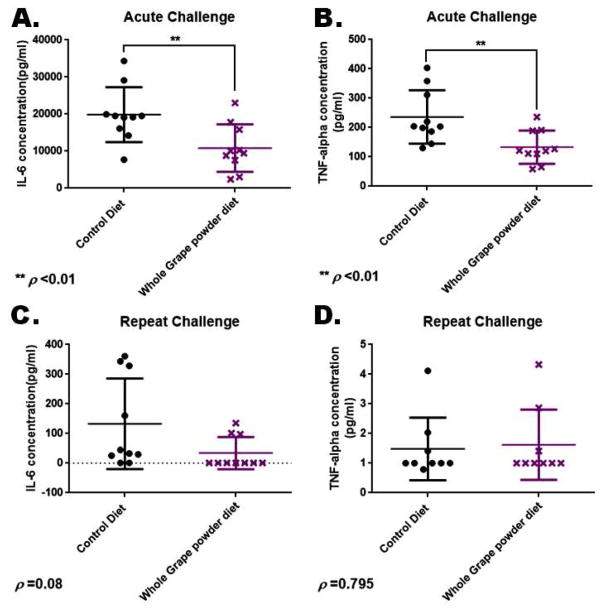
At the end of both acute and repeat-challenge experiments, the mice were sacrificed and terminal blood was collected from each of the animals. Sera were separated from the blood specimen and were analyzed using ELISA for the levels of the inflammatory cytokines IL-6 (NF-κB transcriptional target gene) and TNFα. Data for measurements of IL-6 and TNFα for the acute challenge group are shown in Figure 5A and B, respectively. Data for IL-6 and TNFα measurements from mice in the repeat challenge group are shown in Figure 5C and D, respectively. Analysis of the results showed that the measurements of both IL-6 and TNFα were significantly lower in the animals within the acute challenge groups fed the WGP diet compared to those animals on the control diet. The measurements did not show significant differences in the concentrations of these cytokines in the repeat challenge groups. Given the short time (about 4 hours) before the mice in acute challenge group were sacrificed, it is possible that the low cytokine measurements in this group may actually reflect the already lower pre-challenge steady state levels of these pro-inflammatory cytokines in this group. The repeat challenge mice continued to be challenged over 5 additional days, time sufficiently long enough for the pre-challenge benefits to wane.
Figure 5.
Effect of WGP supplementation on serum levels of the cytokines IL-6 and TNF-α. Terminal sera collected from mice on control or WGP diet were analyzed using ELISA for the levels of IL-6 and TNFα. IL-6 and TNFα data for mice in the acute challenge group are displayed in panels A and B, respectively. IL-6 and TNFα data for mice in the repeat challenge group are shown in panels C and D, respectively. ** statistically significant difference.
4. Discussion and Conclusion
Chronic inflammation is characterized by adaptive and innate immune cells converging on and interacting with the epithelial and mesenchymal cells of the affected organ(s) [42]. These activated inflammatory cells, subsequently induce elevated concentrations of reactive oxygen species (ROS), growth factors and secretion of inflammatory cytokines including interferon (IFN)-γ, interleukin (IL)-2, IL-6 and tumor necrosis factor (TNF)-α [43]. The resulting chronic inflammation adversely affects tissue physiology and morphology, potentially resulting in fibrosis, necrosis, or tissue destruction. These changes in tissue physiology are observed in conditions such as atherosclerosis, ischemic heart disease, cancer, obesity, inflammatory bowel disease, Crohn’s disease, diabetes, and several autoimmune diseases [3, 44–46]. These conditions represent some of the leading causes of death in the United States and worldwide [2].
The purpose of this study was to examine if inclusion of WGP in standard diet would modulate inflammatory signaling in vivo. Comparison was made between animals fed with a control diet supplemented with the principal sugars contained within WGP (i.e. fructose and glucose) and those fed a diet supplemented with WGP. Similar work utilizing WGP as a dietary supplement (as 3–10% dry weight) in inflammatory disease models have been reported within the literature [47–49]. WGP has also been tested to determine the effect of WGP consumption on inflammatory disease markers in human patients [50]. These studies, based upon RNA and protein expression techniques, collectively reported increases in antioxidant enzyme activity and amelioration of disease symptoms when WGP was added to the diet. In our study, we employed a novel approach of using sequential imaging of genetically engineered reporter mice which respond to inflammatory signaling by expressing the enzyme luciferase which can be detected by in vivo imaging techniques.
Our results showed that, when compared to the control diet, WGP supplementation had no effect on the weight dynamics of the animals in this study. It also had no effect on the weight loss resulting from repeat challenge during the last week of the study. These results concur with findings by Patel et al. [47] which showed that WGP supplementation had no effect on the body weight of their experimental animals. On the other hand, recent studies have shown that supplementation of WGP and other grape products in the diet could counteract the adverse effects of high fat diet including adiposity, hyperlipidemia, insulin resistance, and oxidative stress [51–53]; and also reduce inflammatory signaling in the context of cardiac dysfunction [49]. These outcomes are usually attributed to the antioxidant activity of the polyphenolic fraction of grapes and grape products [51].
WGP supplementation significantly slowed down the basal endogenous NF-κB activity in our experimental animals. However, it had minimal or no effect on LPS-induced NF-κB activity, regardless of whether the inflammatory stimulus was a slow-dose repeat challenge or an acute one. Although the repeat challenge data showed a tendency for higher radiance for the WGP group, the overall difference from the control group did not reach a decisive level of statistical significance. Given the borderline significances, these observations prompt further experiments with a different design. On the other hand, animals on the WGP diet had significantly lower serum concentrations of the inflammatory cytokines IL-6 and TNFα in the acutely challenged group. These results are not surprising because WGP reportedly increases IκBα mRNA and protein expression, while downregulating several inflammatory cytokines, including TNFα and TGF-β in cardiac tissue [49]. Since we were not able to collect blood samples before the acute challenge, we could not extricate the effect of the LPS challenge on the serum levels of the inflammatory cytokines. Our experimental design to test the primary hypothesis about the effects of WGP on inflammatory exogenous challenges precluded the sacrifice of mice before challenge. Of note, the NF-κB reporter activity measured as luminescence data did not show significant difference between control and WGP diet groups in the acute challenge group. Therefore, we hypothesize that lower cytokine levels in the acute challenge groups is the result of the low endogenous inflammatory signaling. While reporter activity is an immediate response measureable within the first hours, cytokine response downstream of the NF-κB activation may occur at a later time point.
These data suggest that WGP supplementation seems to have no effect on exogenously induced NF-κB activation by injection of lipopolysaccharide. Multiple factors could be considered as limiting the possible effects of WGP diet on exogenous stimuli, including the length of feeding schedule, the proportion of WGP in the diet, the dose of exogenous stimulus, and organ-specific effects that could not be captured in our setup. Nevertheless, mice fed on WGP-supplemented diets clearly had lower basal levels of inflammatory signaling including reduced cytokines IL-6 and TNFα in the serum.
The overall results from this study suggest that WGP diet reduces basal steady state levels of inflammatory signaling rather than acute or repeat challenges, especially as designed in this study. While persistent low-grade inflammation is a hall mark of many chronic diseases, steady-state basal level of inflammation is also involved in physiological functions such as wound healing. Therefore, further studies that examine the mechanisms and relevance of reduced endogenous inflammatory signaling are recommended.
Highlights.
Whole grape powder in diet decreased basal level of inflammatory signaling
Whole grape powder diet decreased serum TNF-alpha and IL-6 inflammatory cytokine levels
Inclusion of whole grape powder in diet could not ameliorate exogenous inflammatory challenge
Acknowledgments
This study was supported by grants from the California Table Grape Commission, the NIH/NIGMS (grant #SC3109314) and NIH/TU RCMI Core Facility (Grant #G12MD007585). The funding agencies had no influence on the execution, analysis or reporting of the study.
List of abbreviations
- NF-κB
Nuclear Factor kappa-light-chain-enhancer of activated B cells
- WGP
Whole Grape Powder
- LPS
Lipopolysaccharide
- TNFα
Tumor Necrosis Factor alpha
- IL
Interleukin
- ELISA
Enzyme linked immunosorbent assay
- TGF
Transforming growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krishnamurthy VM, Wei G, Baird BC, Murtaugh M, Chonchol MB, Raphael KL, et al. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int. 2012;81:300–6. doi: 10.1038/ki.2011.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson NB, Hayes LD, Brown K, Hoo EC, Ethier KA. CDC National Health Report: leading causes of morbidity and mortality and associated behavioral risk and protective factors--United States, 2005–2013. MMWR Suppl. 2014;63:3–27. [PubMed] [Google Scholar]
- 3.Wu X, Schauss AG. Mitigation of inflammation with foods. J Agric Food Chem. 2012;60:6703–17. doi: 10.1021/jf3007008. [DOI] [PubMed] [Google Scholar]
- 4.Spangenberg EE, Green KN. Inflammation in Alzheimer’s disease: Lessons learned from microglia-depletion models. Brain Behav Immun. 2016 doi: 10.1016/j.bbi.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kung H, Hoyert D, Xu J, Murphy S. Deaths: final data for 2005. National Vital Statistics Reports. 2008:56. [PubMed] [Google Scholar]
- 6.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, et al. Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988–1994 Through 2013–2014. JAMA. 2016;315:2292–9. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–91. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M. Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell. 2010;17:89–97. doi: 10.1016/j.ccr.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meylan E, Dooley AL, Feldser DM, Shen L, Turk E, Ouyang C, et al. Requirement for NF-kappaB signalling in a mouse model of lung adenocarcinoma. Nature. 2009;462:104–7. doi: 10.1038/nature08462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 12.Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–4. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 13.Santoro MM, Samuel T, Mitchell T, Reed JC, Stainier DY. Birc2 (cIap1) regulates endothelial cell integrity and blood vessel homeostasis. Nat Genet. 2007;39:1397–402. doi: 10.1038/ng.2007.8. [DOI] [PubMed] [Google Scholar]
- 14.Garrison JB, Samuel T, Reed JC. TRAF2-binding BIR1 domain of c-IAP2/MALT1 fusion protein is essential for activation of NF-kappaB. Oncogene. 2009;28:1584–93. doi: 10.1038/onc.2009.17. [DOI] [PubMed] [Google Scholar]
- 15.Samuel T, Welsh K, Lober T, Togo SH, Zapata JM, Reed JC. Distinct BIR domains of cIAP1 mediate binding to and ubiquitination of tumor necrosis factor receptor-associated factor 2 and second mitochondrial activator of caspases. J Biol Chem. 2006;281:1080–90. doi: 10.1074/jbc.M509381200. [DOI] [PubMed] [Google Scholar]
- 16.Dmitrieva OS, Shilovskiy IP, Khaitov MR, Grivennikov SI. Interleukins 1 and 6 as Main Mediators of Inflammation and Cancer. Biochemistry (Mosc) 2016;81:80–90. doi: 10.1134/S0006297916020024. [DOI] [PubMed] [Google Scholar]
- 17.Altinoz MA, Korkmaz R. NF-kappaB, macrophage migration inhibitory factor and cyclooxygenase-inhibitions as likely mechanisms behind the acetaminophen- and NSAID-prevention of the ovarian cancer. Neoplasma. 2004;51:239–47. [PubMed] [Google Scholar]
- 18.Chell S, Patsos HA, Qualtrough D, AMHZ, Hicks DJ, Kaidi A, et al. Prospects in NSAID-derived chemoprevention of colorectal cancer. Biochem Soc Trans. 2005;33:667–71. doi: 10.1042/BST0330667. [DOI] [PubMed] [Google Scholar]
- 19.Coghill AE, Phipps AI, Bavry AA, Wactawski-Wende J, Lane DS, Lacroix A, et al. The Association between NSAID Use and Colorectal Cancer Mortality: Results from the Women’s Health Initiative. Cancer Epidemiol Biomarkers Prev. 2012;21:1966–73. doi: 10.1158/1055-9965.EPI-12-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kune GA. Colorectal cancer chemoprevention: aspirin, other NSAID and COX-2 inhibitors. Aust N Z J Surg. 2000;70:452–5. doi: 10.1046/j.1440-1622.2000.01844.x. [DOI] [PubMed] [Google Scholar]
- 21.McCormack VA, Hung RJ, Brenner DR, Bickeboller H, Rosenberger A, Muscat JE, et al. Aspirin and NSAID use and lung cancer risk: a pooled analysis in the International Lung Cancer Consortium (ILCCO) Cancer Causes Control. 2011;22:1709–20. doi: 10.1007/s10552-011-9847-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wernli KJ, Newcomb PA, Hampton JM, Trentham-Dietz A, Egan KM. Inverse association of NSAID use and ovarian cancer in relation to oral contraceptive use and parity. Br J Cancer. 2008;98:1781–3. doi: 10.1038/sj.bjc.6604392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–59. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 24.Enzler T, Sano Y, Choo MK, Cottam HB, Karin M, Tsao H, et al. Cell-selective inhibition of NF-kappaB signaling improves therapeutic index in a melanoma chemotherapy model. Cancer Discov. 2011;1:496–507. doi: 10.1158/2159-8290.CD-11-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuchs O. Transcription factor NF-kappaB inhibitors as single therapeutic agents or in combination with classical chemotherapeutic agents for the treatment of hematologic malignancies. Curr Mol Pharmacol. 2010;3:98–122. doi: 10.2174/1874467211003030098. [DOI] [PubMed] [Google Scholar]
- 26.Yang J, Amiri KI, Burke JR, Schmid JA, Richmond A. BMS-345541 targets inhibitor of kappaB kinase and induces apoptosis in melanoma: involvement of nuclear factor kappaB and mitochondria pathways. Clin Cancer Res. 2006;12:950–60. doi: 10.1158/1078-0432.CCR-05-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J, Splittgerber R, Yull FE, Kantrow S, Ayers GD, Karin M, et al. Conditional ablation of Ikkb inhibits melanoma tumor development in mice. J Clin Invest. 2010;120:2563–74. doi: 10.1172/JCI42358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hideshima T, Neri P, Tassone P, Yasui H, Ishitsuka K, Raje N, et al. MLN120B, a novel IkappaB kinase beta inhibitor, blocks multiple myeloma cell growth in vitro and in vivo. Clin Cancer Res. 2006;12:5887–94. doi: 10.1158/1078-0432.CCR-05-2501. [DOI] [PubMed] [Google Scholar]
- 29.Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–18. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 31.Tapia MA, Gonzalez-Navarrete I, Dalmases A, Bosch M, Rodriguez-Fanjul V, Rolfe M, et al. Inhibition of the canonical IKK/NF kappa B pathway sensitizes human cancer cells to doxorubicin. Cell Cycle. 2007;6:2284–92. doi: 10.4161/cc.6.18.4721. [DOI] [PubMed] [Google Scholar]
- 32.Chuang CC, McIntosh MK. Potential mechanisms by which polyphenol-rich grapes prevent obesity-mediated inflammation and metabolic diseases. Annual review of nutrition. 2011;31:155–76. doi: 10.1146/annurev-nutr-072610-145149. [DOI] [PubMed] [Google Scholar]
- 33.Dohadwala MM, Vita JA. Grapes and cardiovascular disease. J Nutr. 2009;139:1788S–93S. doi: 10.3945/jn.109.107474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kidd PM. Bioavailability and activity of phytosome complexes from botanical polyphenols: the silymarin, curcumin, green tea, and grape seed extracts. Alternative medicine review: a journal of clinical therapeutic. 2009;14:226–46. [PubMed] [Google Scholar]
- 35.Leifert WR, Abeywardena MY. Cardioprotective actions of grape polyphenols. Nutrition research (New York, NY) 2008;28:729–37. doi: 10.1016/j.nutres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Patki G, Allam FH, Atrooz F, Dao AT, Solanki N, Chugh G, et al. Grape powder intake prevents ovariectomy-induced anxiety-like behavior, memory impairment and high blood pressure in female wistar rats. PLoS One. 2013;8:e74522. doi: 10.1371/journal.pone.0074522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perez-Jimenez J, Saura-Calixto F. Grape products and cardiovascular disease risk factors. Nutrition research reviews. 2008;21:158–73. doi: 10.1017/S0954422408125124. [DOI] [PubMed] [Google Scholar]
- 38.Mandel SA, Weinreb O, Amit T, Youdim MB. Molecular mechanisms of the neuroprotective/neurorescue action of multi-target green tea polyphenols. Frontiers in bioscience (Scholar edition) 2012;4:581–98. doi: 10.2741/S286. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez R, Ballester I, Lopez-Posadas R, Suarez MD, Zarzuelo A, Martinez-Augustin O, et al. Effects of flavonoids and other polyphenols on inflammation. Critical reviews in food science and nutrition. 2011;51:331–62. doi: 10.1080/10408390903584094. [DOI] [PubMed] [Google Scholar]
- 40.Accomando S, Pellitteri V, Corsello G. Natural polyphenols as anti-inflammatory agents. Frontiers in bioscience (Scholar edition) 2010;2:318–31. doi: 10.2741/s67. [DOI] [PubMed] [Google Scholar]
- 41.Ghiringhelli F, Rebe C, Hichami A, Delmas D. Immunomodulation and anti-inflammatory roles of polyphenols as anticancer agents. Anti-cancer agents in medicinal chemistry. 2012;12:852–73. doi: 10.2174/187152012802650048. [DOI] [PubMed] [Google Scholar]
- 42.Libby P. Inflammatory mechanisms: the molecular basis of inflammation and disease. Nutr Rev. 2007;65:S140–6. doi: 10.1111/j.1753-4887.2007.tb00352.x. [DOI] [PubMed] [Google Scholar]
- 43.Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg. 2006;391:499–510. doi: 10.1007/s00423-006-0073-1. [DOI] [PubMed] [Google Scholar]
- 44.Ferguson LR, Laing WA. Chronic inflammation, mutation and human disease. Mutat Res. 2010;690:1–2. doi: 10.1016/j.mrfmmm.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 45.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 46.Weiss U. Inflammation. Nature. 2008;454:427. doi: 10.1038/454427a. [DOI] [PubMed] [Google Scholar]
- 47.Patel AK, Davis A, Rodriguez ME, Agron S, Hackam AS. Protective effects of a grape-supplemented diet in a mouse model of retinal degeneration. Nutrition. 2016;32:384–90. doi: 10.1016/j.nut.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chou LM, Lin CI, Chen YH, Liao H, Lin SH. A diet containing grape powder ameliorates the cognitive decline in aged rats with a long-term high-fructose-high-fat dietary pattern. J Nutr Biochem. 2016;34:52–60. doi: 10.1016/j.jnutbio.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 49.Seymour EM, Bennink MR, Watts SW, Bolling SF. Whole grape intake impacts cardiac peroxisome proliferator-activated receptor and nuclear factor kappaB activity and cytokine expression in rats with diastolic dysfunction. Hypertension. 2010;55:1179–85. doi: 10.1161/HYPERTENSIONAHA.109.149393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Janiques AG, de Leal VO, Stockler-Pinto MB, Moreira NX, Mafra D. Effects of grape powder supplementation on inflammatory and antioxidant markers in hemodialysis patients: a randomized double-blind study. J Bras Nefrol. 2014;36:496–501. doi: 10.5935/0101-2800.20140071. [DOI] [PubMed] [Google Scholar]
- 51.Collins B, Hoffman J, Martinez K, Grace M, Lila MA, Cockrell C, et al. A polyphenol-rich fraction obtained from table grapes decreases adiposity, insulin resistance and markers of inflammation and impacts gut microbiota in high-fat-fed mice. J Nutr Biochem. 2016;31:150–65. doi: 10.1016/j.jnutbio.2015.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hernandez-Salinas R, Decap V, Leguina A, Caceres P, Perez D, Urquiaga I, et al. Antioxidant and anti hyperglycemic role of wine grape powder in rats fed with a high fructose diet. Biol Res. 2015;48:53. doi: 10.1186/s40659-015-0045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Castilla P, Echarri R, Davalos A, Cerrato F, Ortega H, Teruel JL, et al. Concentrated red grape juice exerts antioxidant, hypolipidemic, and antiinflammatory effects in both hemodialysis patients and healthy subjects. Am J Clin Nutr. 2006;84:252–62. doi: 10.1093/ajcn/84.1.252. [DOI] [PubMed] [Google Scholar]