Figure 7. Rotavirus-mediated inhibition of interferon induction and amplification.
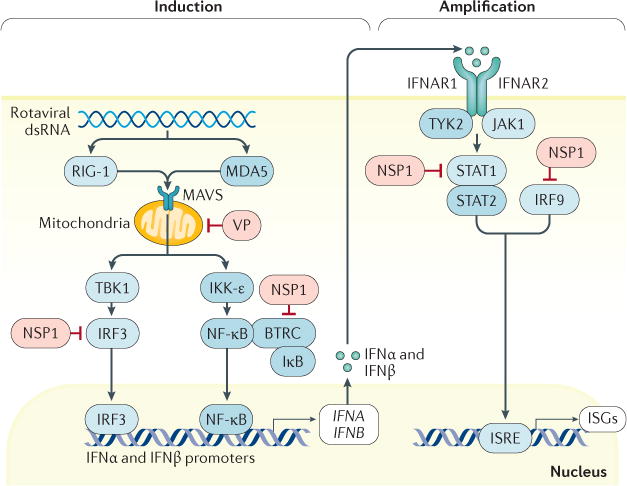
In intestinal epithelial cells, rotaviruses are recognized by the pattern recognition receptors (PRRs) probable ATP-dependent RNA helicase DDX58 (RIG-I) and interferon (IFN)-induced helicase C domain-containing protein 1 (MDA5). These PRRs then signal through mitochondrial antiviral-signalling protein (MAVS) to stimulate the IFN regulatory factor 3 (IRF3) pathway and nuclear factor-κB (NF-κB) pathway to induce IFN type I, II or III responses. In an autocrine manner, this initially stimulates IFN signalling through the IFN receptor and then stimulates signal transducer and activator of transcription (STAT)1, STAT2 and IRF9 to induce transcription of IFN-stimulated genes (ISGs) that amplify IFN production. In human cells in vitro, evasion of the innate IFN response is mediated principally by rotavirus non-structural protein 1 (NSP1) through inhibition of NF-κB activation via degradation of F-box/WD repeat-containing protein 1A (BTRC; also known as β-TrCP)218,219. A second mechanism is mediated by NSP1 and inhibits STAT1 activation, therefore blocking type I and type III IFN responses220. Indeed, results from experiments using human rotavirus strains in human intestinal enteroid cultures185 support the findings that homologous rotavirus infection is very efficient at evading the type I and III IFN responses in mice77. Other viral proteins (VPs) might be involved in the suppression of the IFN response through inactivation of MAVS. IFNAR1, IFN-α/β receptor 1; IκB, inhibitor of nuclear factor-κB; IKK-ε, inhibitor of NF-κB kinase subunit ε; JAK1, Janus kinase 1; ISRE, IFN-stimulated response element; TBK1, TANK-binding kinase 1; TYK2, non-receptor tyrosine kinase TYK2.
