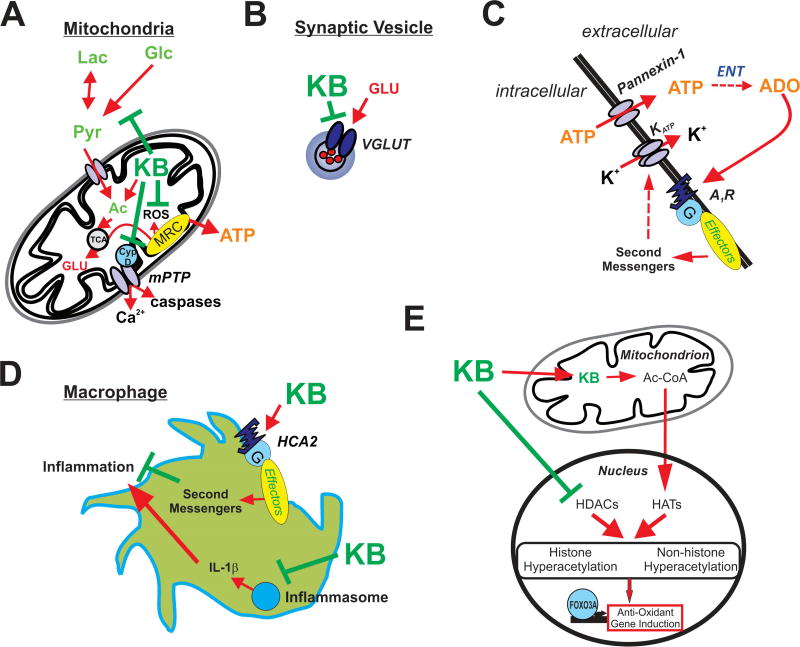
Figure 1.
Illustrations depicting potential mechanisms underlying ketone body-mediated attenuation of CNS hyperexcitability and neuroprotection. (A) Ketone bodies (KB) may enhance ATP production by providing acetyl-CoA (Ac) and inhibit production of reactive oxygen species (ROS) and the mitochondrial permeability transition (mPT) pore, thereby protecting the cell against oxidative injury and preventing excessive release of calcium. (B) KB may inhibit vesicular glutamate transporters (VGLUT), decreasing the amount of glutamate loaded in vesicles and reducing the size of glutamate quanta released during synaptic transmission. (C) KB-mediated increases in intracellular ATP and subsequent release through pannexin channels lead to adenosine (ADO) synthesis via ectonucleotidases (ENT) in the extracellular space. ADO in turn binds to inhibitory adenosine type 1 receptors (A1Rs) which are coupled to the indirect opening of KATP channels. (D) KB activate HCA2 receptors and inhibit the assembly of the NRLP3 inflammasome; thus, KB attenuate inflammatory mediators produced by infiltrating macrophages. (E) KB also promote histone and non-histone hyperacetylation by increasing acetyl-CoA, a substrate for histone acetyltransferases (HATs), and directly inhibiting histone deacetylases (HDACs) – with the end result of increasing endogenous anti-oxidants (among other actions).