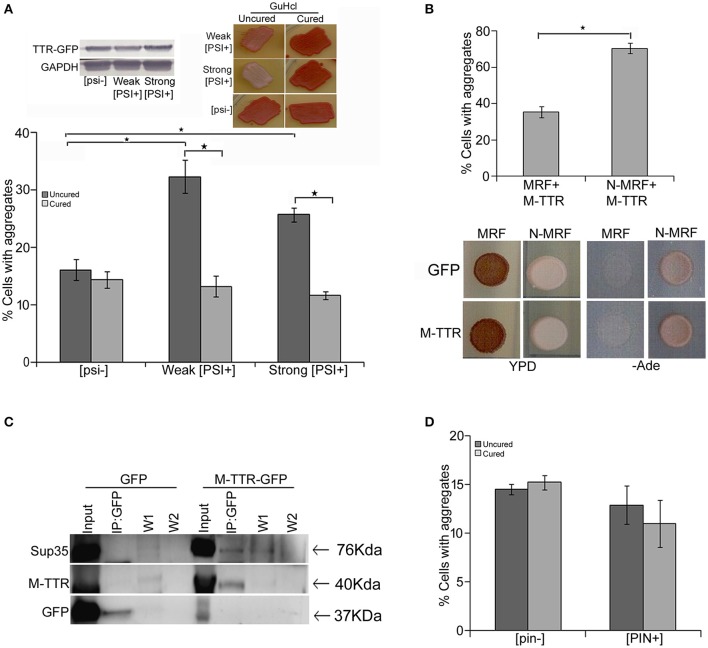
Figure 3.
Effect of endogenous prion strains on M-TTR aggregation. (A) The effect of endogenous prion, [PSI+], on M-TTR aggregation was analyzed by manually counting percentage of cells with M-TTR-GFP aggregates (under the microscope) in [psi−] (non-prion) and weak and strong variants of [PSI+], depicted as uncured strains in the graph. Left inset: Equal levels of M-TTR-GFP protein in different strains were determined by immunobloting using anti-GFP antibody. M-TTR-GFP aggregation was again determined after curing the [PSI+] prion in these strains by passaging on GuHCL (right inset), depicted as cured in the graph. Error bars represent standard errors of the mean of three independent transformants. The significant difference in aggregation between [PSI+] variants and [psi−] as well as between uncured and cured strains was assessed by using two-tailed t-test (*depicts p-value < 0.05). (B) Upper panel: Percentage of cells with M-TTR-GFP aggregates were analyzed in a [PSI+] sup35Δ ade1-14 strain maintained by either expression of full length (N-MRF) or the functional domain (MRF) lacking the prion (N) domain of Sup35. The strain expressing MRF could not propagate [PSI+]. Aggregation counting for all the experiments was done for atleast three independent transformants for each sample. Error bars represent standard errors of the mean of triplicates. To assess the significant difference in M-TTR-GFP aggregation between N-MRF and MRF expressing cells, two-tailed t-test was performed (*depicts p-value < 0.05). Lower panel: The effect of M-TTR-GFP in curing [PSI+] prion was analyzed by co-overexpressing M-TTR-GFP and N-MRF in [PSI+] sup35Δ ade1-14 and spotting on rich media (YPD) and media lacking adenine (-Ade). Cells were examined for any change in coloration from white/pink (prion) to red (non-prion) on rich media and growth on -Ade media. M-TTR-GFP co-expressed with MRF (soluble functional domain of Sup35) and GFP alone co-expressed with MRF as well as N-MRF were used as controls. (C) Direct interaction of heterologous aggregates was examined by pull down of Sup35 with M-TTR-GFP aggregates. A weak [PSI+] strain overexpressing M-TTR-GFP fusion protein were lysed and incubated with anti-GFP antibody conjugated beads in a column. Cells overexpressing GFP were used as a control. Total protein of M-TTR-GFP and GFP lysates were normalized before incubation with the anti-GFP beads. The incubation complexes were washed twice (W1 and W2) and co-precipitated protein was eluted (IP:GFP) and resolved on 10% SDS-PAGE. Blot was probed with anti-GFP and anti-Sup35 (BE4) antibodies. Input fraction is 100 μg of the total protein lysates. (D) The effect of endogenous yeast prion [PIN+] on M-TTR aggregation was also analyzed by counting the percentage of cells with M-TTR-GFP aggregates in [pin−] (non-prion) and [PIN+] (prion) form of Rnq1. Aggregation counting was done for three independent transformants for each. Error bars represent standard errors of the mean of triplicates. The significance of difference in M-TTR-GFP aggregation between [PIN+] and [pin−] samples was analyzed by using two-tailed t-test (*depicts p-value < 0.05).