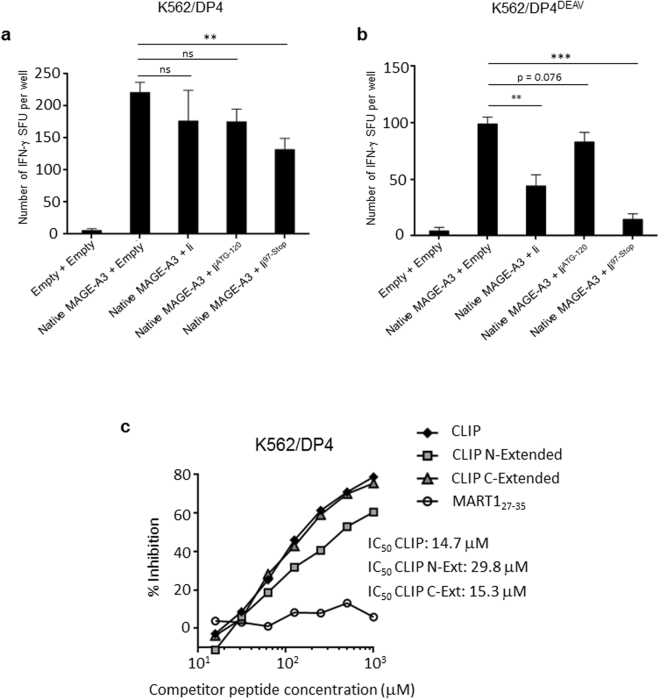
Figure 6.
The N-terminal Ii region(s) inhibit CLIP-dependent binding to DP4 in vivo and in vitro. (a,b) DP4/MAGE-A3-specific CD4+ T cells were stimulated using the indicated K562-based aAPCs, and IFN-γ responses were measured by ELISPOT analysis. K562 cells stably expressing DP4 (a) or DP484DEAV87 (b) were transiently transduced with either the empty vector or full-length native MAGE-A3, and one of wild-type Ii, IiATG-120, Ii97-Stop, or the empty vector as indicated and used to stimulate T cells. The transient transfection efficiencies were normalized to EGFP expression as measured by flow cytometry (Supplementary Figure 3). The data shown represent the mean ± SD of experiments performed in triplicate. The results are representative of at least three independent experiments. ns: not significant; **p < 0.01, ***p < 0.001 in unpaired, two-tailed Welch’s t-test. (c) A K562 cell-based competitive binding assay was performed using K562 cells stably expressing DP4. The cells were pulsed with graded concentrations of the CLIP, N-extended CLIP (by five amino acids), C-extended CLIP (by five amino acids), or MART127–35 peptide in the presence of 2 μM of the biotin-conjugated reference peptide HIV ENV31–45. Then, the cells were stained with phycoerythrin-conjugated streptavidin and analyzed by flow cytometry. The percent inhibition of binding by the CLIP peptide was calculated as the mean fluorescence intensity (MFI) using the formula: %inhibition = [1 − (MFI with CLIP peptide/MFI without CLIP peptide)] × 100.