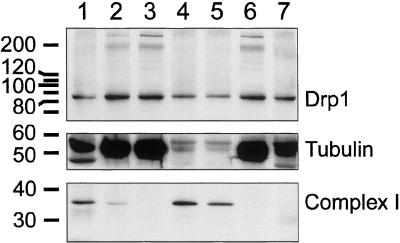
Figure 5.
Localization of Drp1 determined by subcellular fractionation of bovine brain. Lane 1, crude extract; lane 2, postnuclear supernatant (S1); lane 3, medium speed supernatant (S2); lane 4, medium-speed pellet (P2); lane 5, mitochondrial fractions after further purification on a Percoll gradient; lane 6, high-speed supernatant (S3); and lane 7, high-speed pellet (P3). The subcellular fractions were probed with antibody for Drp1, for a cytosolic marker (tubulin), and for mitochondria (39-kDa subunit of OxPhos complex I). The number of volume equivalents needed to load equal amounts of protein (75 μg/lane) was 1× for lane 1, 3× for lane 2 and 3, 43× for lane 4, 21× for lane 5, 3× for lane 6, and 75× for lane 7. Densitometry and adjustment for volume equivalents shows that ∼3% of Drp1 is in the mitochondrial fractions (lanes 4 and 5), 2% is in the high-speed pellet and the rest is in the supernatant. This fractionation experiment was replicated five times, each time giving similar results.