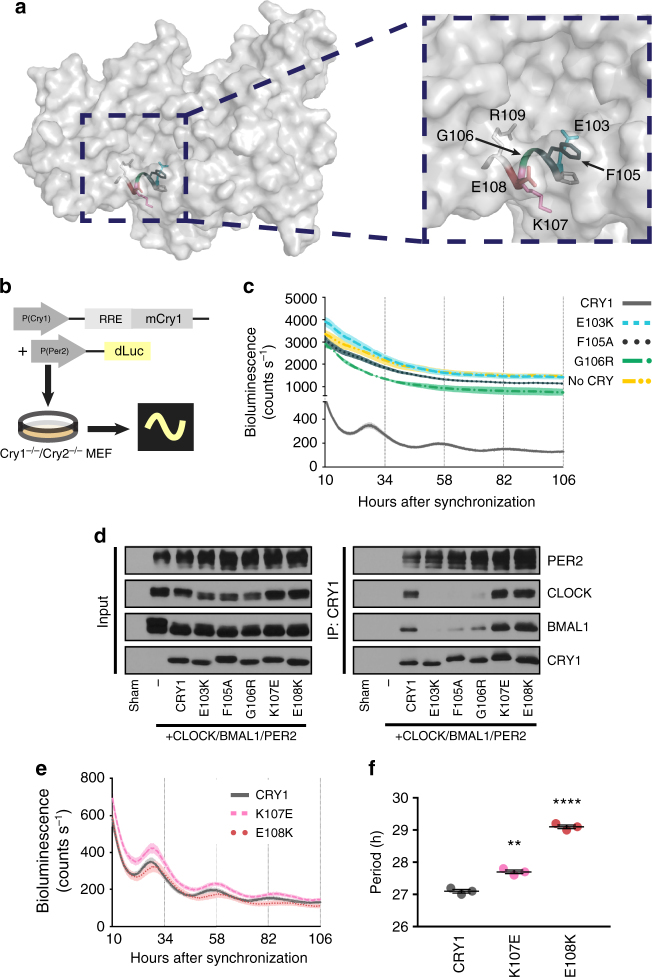
Fig. 3.
The lower helical boundary of CRY’s secondary pocket gates interaction with CLOCK. a A surface view of the structure of CRY1 (PDB: 5T5X) with the lower helix of the secondary pocket shown as a ribbon diagram with side chains shown as sticks and relevant residues color-coded for the rest of the figure. Inset is a magnified view of this helix with residues labeled. b Schematic of Cry1 rescue assay. RRE is an ROR response element necessary for delayed expression of CRY. c Rescue assays with CRY1 mutants identified in the SCA (n = 3 per condition, reflective of 6 plates from two independent experiments) shown as means ± SEM. d Co-IP assay with PER2, CLOCK, BMAL1, and various CRY1 mutants. Multiple bands in PER2, CLOCK, and BMAL1 lanes are indicative of post-translational modifications on these proteins. Blot is representative of at least three independent experiments. e Rescue assays for two CRY1 residues not identified in the SCA (n = 3 per condition, reflective of 6 plates from two independent experiments) shown as means ± SEM. f Period plot for the data shown in e. Asterisks show significance by unpaired t test with Welch’s correction (**p = 0.0018, ****p < 0.0001)