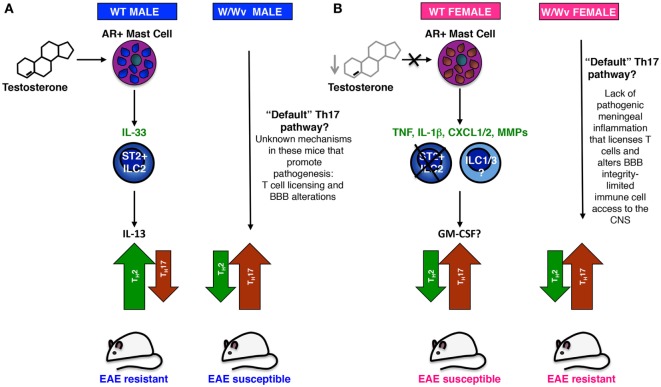
Figure 1.
A model of sex-dimorphic T helper (Th) responses in experimental autoimmune encephalomyelitis (EAE) informed by studies in KitW/Wv mice. (A) Testosterone-dependent IL-33 production in androgen receptor+ (AR+) mast cells promotes a non-pathogenic Th2 anti-myelin response in PLP139–151 immunized wild-type SJL males. Early IL-33 production by mast cells (and perhaps other AR+) cells activates ST2+ innate lymphoid cells (ILC2s), which in turn express IL-13, a cytokine that polarizes the response to one that is Th2-dominant. This Th2 polarization appears to take place during priming in the secondary lymphoid organs and is likely maintained in Th2 effector cells by resident ILC2s in the meninges and Central Nervous System (CNS). Testosterone potentially acts in two ways: (1) acute increases in systemic testosterone directly activate mast cells, and perhaps other AR+ cells, resulting in increased IL-33 expression; (2) long-term testosterone exposure may also exert effects on the Il33 chromatin landscape in mast cells, enabling higher potential for activation-induced expression. In the absence of IL-33-producing mast cells and ILC2s, a major but not exclusive IL-33 target cell, male KitW/Wv mice cannot generate a strong Th2 response and “default” to a pathogenic Th17 response. In addition to anti-myelin-specific Th17 cells, unknown mechanisms promote inflammatory cell influx to the CNS and promote disease susceptibility in these mice. (B) Immunized wild-type females “default” to a Th17 response because they lack sufficient testosterone to elicit the IL-33–ILC2–Th2 pathway. Low testosterone may fail to acutely induce IL-33, but may also affect the Il33 chromatin landscape, lessening the potential for mast cell IL-33 expression. Upon activation female-derived mast cells express an alternative set of more pro-inflammatory effector molecules. IL-1β-producing mast cells “license” these T cells as they transit through the meninges by eliciting GM-CSF production and enhancing encephalitogenicity. Inflammatory cell influx to the CNS is facilitated by mast cell TNF, CXCL1/2, and matrix metalloprotease (MMP) production that recruits neutrophils and degrades the extracellular matrix, altering blood–brain barrier (BBB) integrity. Increased ILC1 and ILC3 activity in females may also facilitate meningeal inflammation and immune cell infiltration to the CNS (73). Resistant female KitW/Wv mice also generate a Th17 response, but in the absence of meningeal inflammation and T cell licensing, driven by mast cell-derived TNF, IL-1β, CXCL1/2, and MMPs, these cells have only limited access to the CNS parenchyma.