Fig. 1.
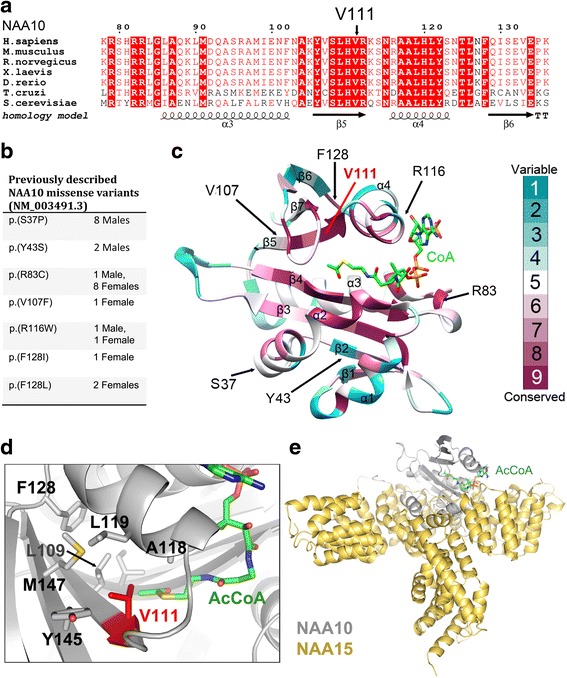
NAA10 multiple sequence alignment and structural conservation. a NAA10 multiple sequence alignment showing amino acids 78–137 (of human NAA10) (b) Previously described NAA10 variants identified in patients with ID/DD. c Cartoon representation of NAA10 colored with respect to evolutionary conservation. More conserved regions are colored in dark magenta, less conserved regions are colored in dark cyan. V111 is located towards the end of β5 and is highly conserved throughout evolution. d The side chain of V111 is pointing towards a hydrophobic pocket together with Y145, M147, L109 and L119. V111 is also located in close proximity to the sulfur-acetyl group of AcCoA, and to the β6-β7 loop region that is very important for peptide substrate binding. e Homology model of the human NatA complex. The auxiliary subunit NAA15 is shown in yellow cartoon, the catalytic subunit NAA10 is shown in white cartoon and the AcCoA is shown as green sticks
