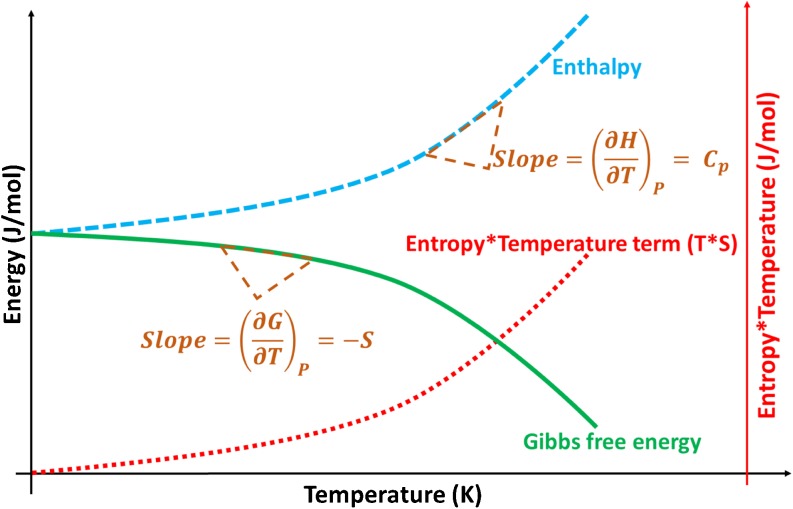
Fig. 1.
Diagram showing the variation of Gibbs free energy, enthalpy, and entropy with temperature for a crystalline solid material. The slope of the enthalpy curve gives the value of heat capacity and the value of the Gibbs free energy slope gives the value of entropy. For pharmaceuticals, the importance of pressure is usually omitted, since the operations are performed in conditions where the influence of pressure is considered negligible