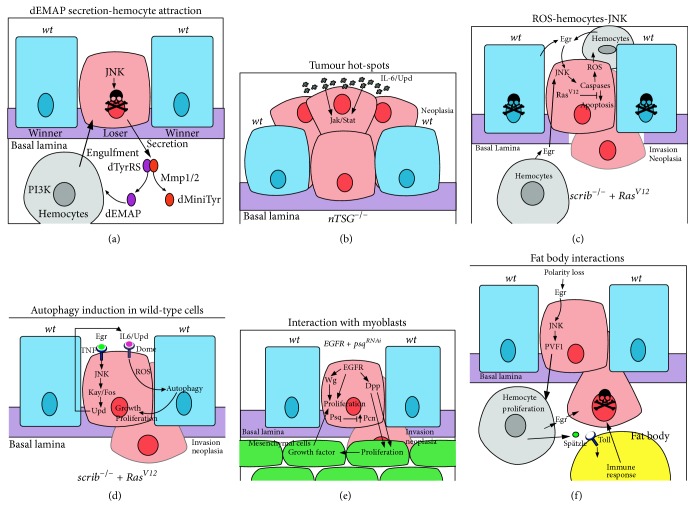
Figure 2.
Cooperative interactions between the tumour and surrounding cells in tumorigenesis. Interactions between cells are shown that result in either the death of the mutant cell or cell survival, proliferation, and neoplastic transformation. Mutant cells are in pink, wild-type cells are in blue, hemocytes are in grey, myoblasts (mesenchymal cells) are in green, a fat body adipocyte is in yellow, and the basement membrane (basal lamina) is in purple. (a) dEMAP secretion-hemocyte attraction: JNK signalling in a cell polarity-impaired loser cell transcriptionally upregulates MMP1, which acts to cleave secreted dTyrRS to form dEMAP and dminiTyr. dEMAP attracts hemocytes to the loser cell by upregulating PI3K signalling in the hemocytes, which is required for chemotaxis and possibly engulfment of the loser cell. (b) Tumour hot-spots: neoplastic tumour-suppressor mutants (nTSGs) induce tumours more preferably, in regions where there is a stiff basal lamina and there are developmentally high levels of the Upd (IL-6) ligand to elevate Jak-Stat signalling, which promotes cell survival and proliferation of the tumour cells. (c) ROS-hemocytes-JNK: in scrib mutant RasV12-expressing tumour cells, a feedback loop between the hemocytes and the mutant cells promotes tumorigenesis. In the mutant cells, Ras signalling and caspase activation leads to ROS production that is released from the cells and promotes hemocytes to produce Egr (TNF). Egr signals via the TNFR-JNK pathway in the mutant cell leading to the upregulation of caspase activity, and some apoptosis, which is required for tumour overgrowth and invasion. Due to the disruption of the peripodial epithelium in large scrib mutant RasV12-expressing tumours, hemocytes most likely interact with the tumour on both apical and basal sides. (d) Induction of autophagy in surrounding wild-type cells: scrib mutant RasV12-expressing tumour cells are metabolically stressed, which leads to ROS production. Egr-JNK signalling leads to the transcriptional upregulation of Upd, ligands for the Dome-Jak-Stat signalling pathway, which is elevated in the mutant cells. Jak-Stat signalling and ROS production are required for the induction of autophagy in the surrounding wild-type cells, and also at distant sites, such as the fat body, muscle, and gut (not shown), which facilitates tumour growth and neoplastic transformation, possibly through supplying amino acids, glucose, and other nutrients to the tumour cells. (e) Interactions with myoblasts: in EGFR-overexpressing psq-knockdown tumours cooperative interactions are observed between the tumour cells and the surrounding myoblasts (mesenchymal cells). EGFR induces Wg and Dpp expression, and psq knockdown leads to increased levels of the extracellular matrix protein, Perlecan (Pcn). Wg acts to promote proliferation of the tumour cells, whilst Dpp, facilitated by Pcn in the basal lamina, stimulates proliferation of the myoblast cells. In turn, the myoblast cells provide unidentified growth factors that drive proliferation and neoplastic transformation of the tumour cells. Myoblasts also supply Egr (not shown), which would be expected to activate the TNFR-JNK signalling pathway in the tumour cells. (f) Interactions with the fat body: polarity-impaired tumours through Egr-JNK signalling upregulate PVF1, a ligand for the PVR receptor on hemocytes, which promotes hemocyte proliferation. Hemocytes, in turn, supply Egr to the tumour cells, and the Toll Receptor ligand, Spätzle, to the fat body, which induces innate immune system signalling in the fat body. These interactions are required to induce apoptosis of tumour cells.