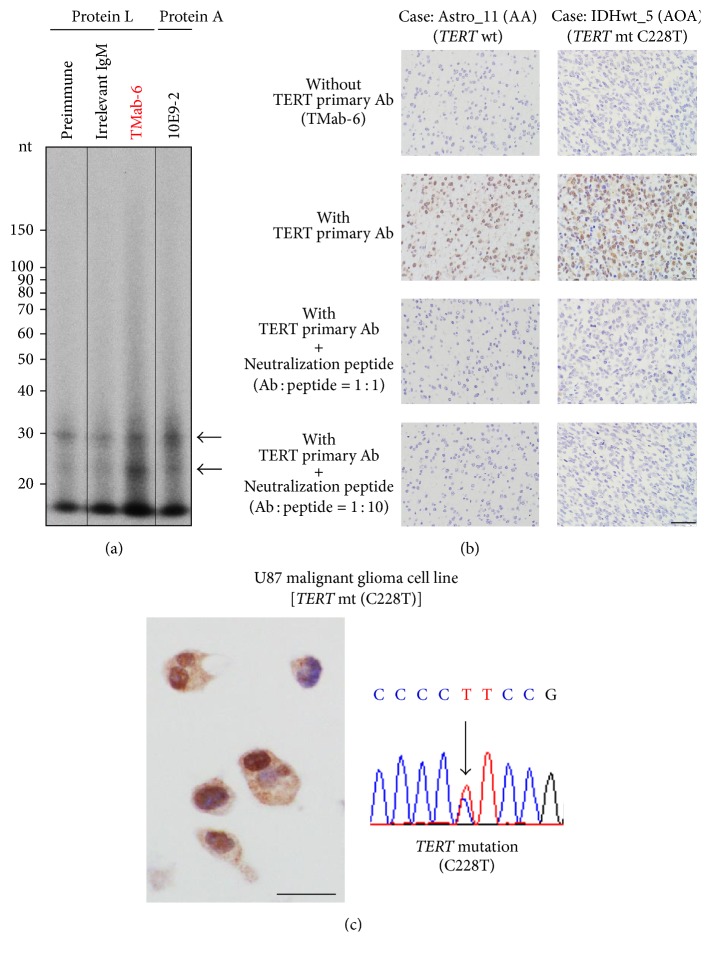
Figure 2.
Validation of a newly developed TERT-specific antibody (TMab-6) usable for human glioma tissue. (a) Endogenous TERT was immunoprecipitated with an anti-human TERT mAb (TMab-6) followed by an RNA-dependent RNA polymerase (RdRP) assay using HeLa cells treated with nocodazole. Arrows indicate RdRP products. The preimmune (without antibodies) and irrelevant IgM lanes are a negative control, and the 10E9-2 antibody (MBL, Nagoya, Japan) lane is a positive control. nt, nucleotide. (b) The IDH-mutant astrocytoma (anaplastic astrocytoma: AA) and IDH-wildtype diffuse astrocytoma (Histologically anaplastic oligoastrocytoma: AOA) cases were analyzed for TERT protein expression. TMab-6 specifically recognized the nuclei of the tumor cells, and immunoreactivity was not detected in the negative control section without the application of TMab-6. An antibody absorption test with synthetic neutralization peptide of TERT further validated the specificity of TMab-6 as an anti-TERT antibody which is applicable to human glioma tissue. (c) TMab-6 Immunostaining of U87 malignant glioma cell lines with a TERT hotspot mutation (C228T) showed strong nuclear immunoreactivity for TERT. Ab, antibody; mt, mutation. Scale bar = 40 μm.