Figure 3.
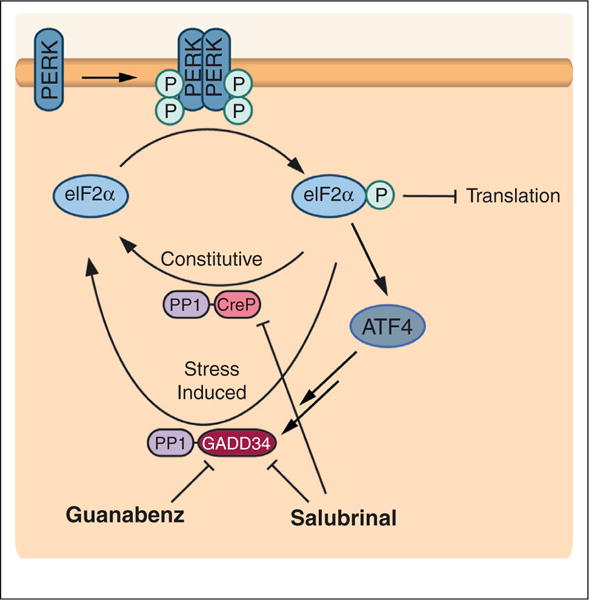
Small molecule modulation of PERK signaling can be mediated by targeting eIF2α phosphatase complexes. PERK activation increases eIF2α phosphorylation, which in turn attenuates translation and increases expression of stress-responsive transcription factors. This pathway is negatively regulated by a phosphatase complex between protein phosphatase 1 and the constitutively expressed regulatory subunit CreP (PP1–CreP) and/or a stress-induced regulatory subunit GADD34 (PP1–GADD34). Small molecules that target these complexes can modulate eIF2α-dependent signaling, effectively mimicking PERK activation. Salubrinal inhibits both the PP1–CreP and PP1–GADD34 phosphatase complexes, allowing for increased eIF2α phosphorylation in the absence of stress. Guanabenz selectively targets PP1–GADD34, providing a mechanism to prolong PERK-dependent eIF2α phosphorylation signaling activation in response to ER stress. Figure adapted from Wiseman et al. [39].
