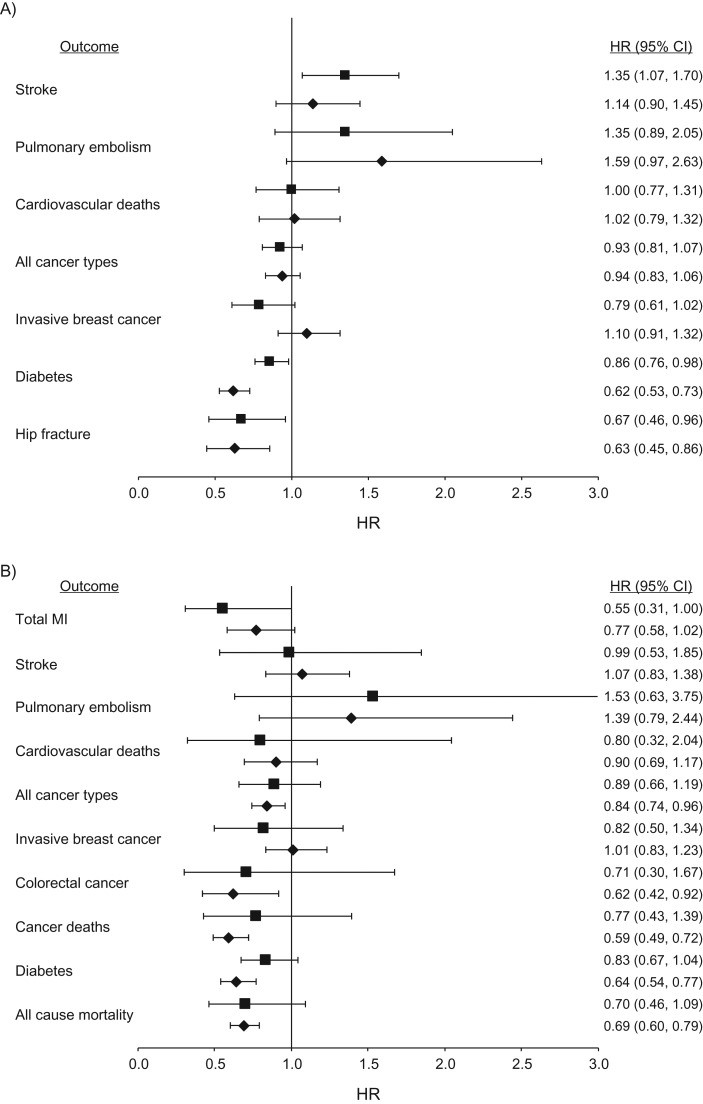
Figure 1.
Association between hormone therapy use (oral conjugated equine estrogens (CEE) (0.625 mg/day)) and health outcomes among postmenopausal women who underwent a hysterectomy, in the Nurses’ Health Study (1980–2002) (diamonds) and in the intervention phase of the Women's Health Initiative (WHI) (squares) CEE-alone trial (1) among participants aged 50–79 years (A) and aged 50–59 years (B) at entry into analysis. All NHS models adjusted for age, calendar time, smoking status, alcohol intake, physical activity, body mass index, aspirin use of at least 1 day/month, history of high blood pressure, history of hypercholesterolemia, history of type 2 diabetes mellitus (all models except diabetes as an outcome), age at menopause, parental history of early myocardial infarction (MI), parental history of cancer, duration of CEE use, and duration of CEE plus medroxyprogesterone acetate (MPA) use. Breast cancer models additionally adjusted for height, parity, age at first birth, body mass index at age 18, history of benign breast disease, and mammogram in the previous cycle. Results for total MI, colorectal cancer, cancer deaths, and all-cause mortality are not reported for the age group 50–79 years because a significant interaction by age was reported in the intervention phase of the WHI (1). Hip fracture results are shown only for the 50–79 years group because there were fewer than 5 cases in the WHI in the 50–59 years group (1). CI, confidence interval; HR, hazard ratio.