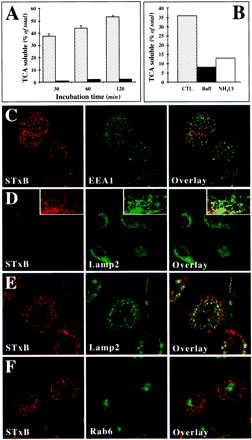
Figure 4.
Analysis of STxB transport to the late endocytic pathway in macrophages and DC. (A) Iodinated STxB-Glyc-KDEL (50 nM; 2.5 μg/ml) was incubated for 30 min at 37°C with macrophages (speckled columns) or bound to HeLa cells (▪) on ice (A). The cells were then washed on ice, chased at 37°C for the indicated times, and TCA-soluble counts (percentage of the total cell associated radioactivity) were determined. Means (±SEM) of three independent experiments are shown. The appearance of TCA-soluble counts in macrophages indicated that in these cells, STxB was transported to late endosomes/lysosomes. (B) Experiment on macrophages as described in A, in which during the chase period of 30 min, 1 μM Bafi and 50 mM ammonium chloride (NH4Cl) were added. (C) STxB internalized at 19.5°C colocalized extensively with the early endosomal protein EEA1. STxB was continuously internalized for 1 h. The cells were then fixed and stained with the indicated antibodies. (D) After a 30-min shift to 37°C after internalization at 19.5°C, STxB appeared to be lost from the cells. (Insets) Enhancement of the remaining signal showed a limited codistribution of the remaining STxB labeling with the lysosomal glycoprotein Lamp2, suggesting that STxB might be targeted to lysosomes for degradation. (E) A 30-min shift to 37°C in the presence of Bafi allowed detecting a significant degree of codistribution between STxB and Lamp2. (F) As in E, macrophages were shifted to 37°C in the presence of Bafi and then stained for the indicated proteins. Note that even when STxB degradation was prevented, no significant accumulation in the Golgi region marked by Rab6 could be detected.