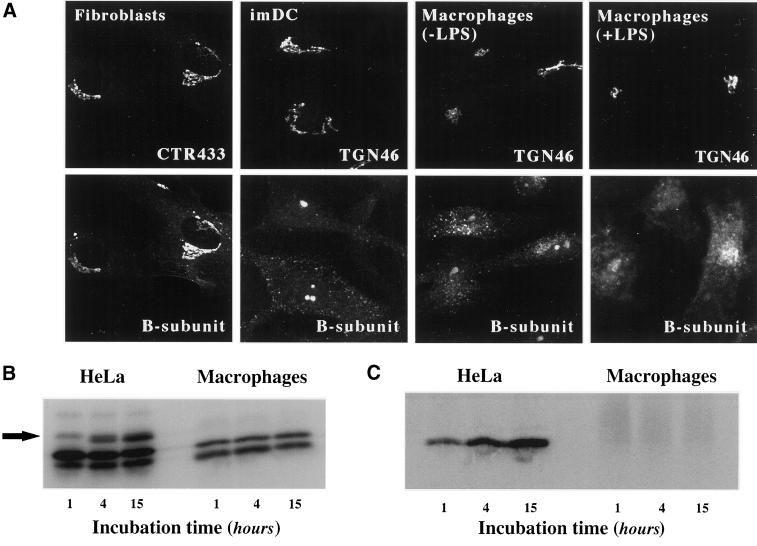
Figure 5.
Analysis of STxB transport to the biosynthetic/secretory pathway in macrophages and DC. (A) Immunofluorescence analysis of STxB transport in different primary human cells. Wild-type STxB was internalized constitutively for 45 min at 37°C into macrophages and imDCs, and after binding into fibroblast and LPS-treated macrophages. The cells were then fixed and stained for STxB (bottom) and the Golgi marker CTR433 or the TGN marker TGN46 (top). In macrophages and imDCs, STxB was detected in vesicular cytoplasmic structures, but not in the TGN, whereas in fibroblasts, STxB readily entered the TGN. In LPS-treated macrophages, STxB accumulated in a number of cytoplasmic locations that only partly covered the Golgi region. (B) Glycosylation analysis. Iodinated STxB-Glyc-KDEL (50 nM; 2.5 μg/ml) was internalized after prebinding into HeLa cells or continuously into macrophages for the indicated times. At the end of each incubation period, the cells were lysed and lysates were analyzed by gel electrophoresis. The same numbers of counts were loaded on gels. The arrow indicates the glycosylation product, i.e., ER-localized STxB, which was observed in HeLa cells, but not in macrophages. The lowest bands are proteolytical cleavage products. (C) Sulfation analysis. 0.2 μM of the fusion protein STxB-Sulf2 (10 μg/ml) was internalized after prebinding into HeLa cells or continuously into macrophages for the indicated times in the presence of radioactive sulfate. The cells were then lysed, STxB was immunoprecipitated, and immunoprecipitates were analyzed by gel electrophoresis. Sulfated STxB-Sulf2, i.e., protein that had passed through the TGN, was detected in HeLa cells, but not in macrophages.