Figure 1.
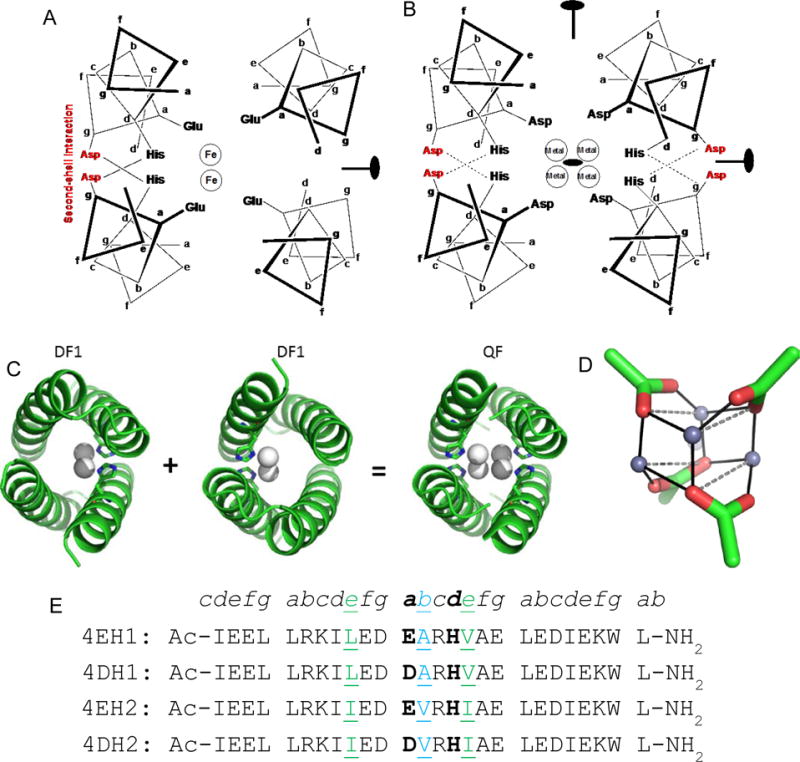
Design of tetranuclear clusters in helix bundles. (A) Previously designed diiron protein DF1 has 4 Glu and 2 His residues with 2 metal ions bound positioned off of the superhelical axis. The positions of second-shell Asp and the C2 symmetry axis are also indicated. (B) D2-symmetrical DF1 has four Asp and four His residues coordinating 4 ions at the core. The positions of the three orthogonal two-fold axes are indicated. Second-shell Asp residues at an interfacial g position (shown in red) are also included to interact with the first-shell Asp ligands. (C) Crystal structure of dimeric DF1, showing the displacement of the two metal ions away from the central bundle axis towards the His ligands. Two orientations of DF1 can be conceptually combined to create a structure that binds four metal ions. (D) Designed tetranuclear binding site, showing the positions of the carboxylates and Zn2+ ions. (E) Designed peptide sequences are shown with heptad repeat designation. The peptides are N-acetylated and C-amidated. The primary ligands are in bold. Residues at positions b and e, which were varied to probe the effect of interhelical packing on the structure of the metal-binding site are shown in green.
