Table 1.
Structures of sEH inhibitors commonly in research, and three compoundsα used in clinical trials.
| Compound* | Structure | IC50 for human sEH# | Comments |
|---|---|---|---|
| AUDA, UC700 |
|
3nM <11nM> |
Early sEH inhibitor with high lipophilicity and high melting point designed to mimic the 14, 15-EET structure. It is also a PPAR alpha agonist and it would be expected, from its structure, to be biologically active as an EET mimic. All lipophilic high melting sEH inhibitors are only available biologically in true solution in an organic co-solvent. |
| AEPU, UC950 |
|
1nM <5nM> |
A water miscible sEH inhibitor with low melting point, and activity on the sEH from many species. Rapidly penetrates cell membranes, protein binding is low, and it was designed for rapid metabolism |
| t-TUCB, UC1728 |
|
900pM <1nM> |
A lipophilic sEH inhibitor with broad potency across many species. Improved pharmacokinetics compared with AUDA due to removal of the metabolically labile adamantane group. Commonly used in equine and companion animal studies. Lipophilic and high melting point. |
| TPPU, UC1770 |
|
600pM <10nM> |
Widely used and commercially available sEH inhibitor of moderate water solubility. A lipophilic and high melting point compound, which must be administered in an organic co-solvent. High target occupancy. Currently the most commonly used model sEH inhibitor. Most piperidines have low potency outside of primate and rodent species. |
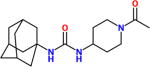
| AR9281α, APAU, UC1154 |
|
15nM <6nM> |
No off-target effects seen in Phase I and II clinical trials in hypertensive – pre-diabetic patients run by Arête Therapeutics. More water soluble than most piperidine sEH inhibitors. Very short half-life in most species. It shows poor target occupancy. Like UC950, it is an excellent tool if a short half-life water soluble compound is needed. |
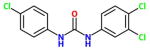
| TCCα |
|
13 nM <21nM> {353nM} |
Triclocarban is an old topical antimicrobial commonly used in cosmetics and hard soaps. It is lipophilic and has a short half life due to N-glycosylation. It was successful in a double blind 30 person clinical trial as a topical with diclofenac with diabetic neuropathy. Note the very poor activity on murine enzyme. The study was run by a collaboration between Sphaera and Synthia Dermatec. |
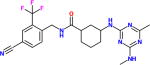
| GSK2256294α |
|
400pM [27pM] |
A lipophilic high melting compound run in three Phase I trials, and with some efficacy data on cigarette induced pulmonary disease. Good pharmacokinetics and no adverse effects noted. It is available for experimental use. The pM potency was reported by GSK using a different substrate. |
Several synonyms are used in the literature for the compounds.
IC50 can be highly reproducible but the value depends upon the substrate structure and its concentration as well as other incubation conditions. Unless otherwise noted by <>, {} or [] these values were determined with the spectral substrate CMNPC and the pure human recombinant sEH. Many sEH inhibitors are so powerful that they violate Michaelis-Menton assumptions. Most are transition state mimics showing slow – tight binding kinetics so that the target occupancy driven by kinetic koff appears the best predictor of in vivo efficacy. IC50 on the rat is noted by <>, and IC50 on the mouse is noted by {}.
