Abstract
Insulin-secreting β-cells are heterogeneous in their regulation of hormone release. While long known, recent technological advances and new markers have allowed the identification of novel subpopulations, improving our understanding of the molecular basis for heterogeneity. This includes specific subpopulations with distinct functional characteristics, developmental programs, abilities to proliferate in response to metabolic or developmental cues, and resistance to immune-mediated damage. Importantly, these subpopulations change in disease or aging, including in human disease. Although discovering new β-cell subpopulations has substantially advanced our understanding of islet biology, a point of caution is that these characteristics have often necessarily been identified in single β-cells dissociated from the islet. β-Cells in the islet show extensive communication with each other via gap junctions and with other cell types via diffusible chemical messengers. As such, how these different subpopulations contribute to in situ islet function, including during plasticity, is not well understood. We will discuss recent findings revealing functional β-cell subpopulations in the intact islet, the underlying basis for these identified subpopulations, and how these subpopulations may influence in situ islet function. Furthermore, we will discuss the outlook for emerging technologies to gain further insight into the role of subpopulations in in situ islet function.
Introduction to β-Cell Heterogeneity
A β-cell is a terminally differentiated cell that produces and secretes insulin in a glucose-regulated manner. Importantly, β-cells have the ability to adapt to changes in metabolic demand through increased insulin secretion and/or number. In most vertebrate species, β-cells form clusters with other hormone-secreting cells (glucagon-secreting α-cells, somatostatin-secreting δ-cells) within islets of Langerhans. Very early studies of the β-cell assumed them to be homogenous based on a lack of morphological differences. However, detailed studies subsequently determined that there exists a broad heterogeneity in the function of β-cells. These early studies of β-cell heterogeneity are summarized by the landmark review of Pipeleers (1), which describes with remarkable foresight the presence, characteristics, and role of functional β-cell subpopulations. This includes how dissociated β-cells show functional heterogeneity, with populations of cells displaying higher levels of glucose metabolism, redox state, insulin synthesis, membrane potential, and insulin secretion; that morphological markers (nuclear size, insulin granularity) can differentiate β-cell subpopulations with differing glucose sensitivity and insulin secretion levels; that β-cells show heterogeneous expression of key proteins such as glucokinase (GCK), connexins, or insulin, including spatial variations across the islet; that β-cells with low glucose-stimulated insulin secretion preferentially increase in number under development or metabolic stress; and that β-cells vary in their sensitivity to cytotoxic agents. Despite this in-depth knowledge, there have been several gaps in our understanding that have persisted until recently:
What is the molecular basis for β-cell functional diversity?
Which markers can be used to identify and characterize β-cell subpopulations?
Does functional heterogeneity in the intact islet or pancreas mirror that observed among dissociated β-cells?
What is the role of β-cell heterogeneity in islet function and glucose homeostasis, and can changes in heterogeneity contribute to diabetes?
Are β-cells fixed in specific functional states, or can they transition between states over time?
We will describe recent technological advances and studies that have answered some of these key questions, with a focus on understanding the consequence of heterogeneity in β-cell function within the islet setting.
Recent Advances Characterizing β-Cell Heterogeneity
Early and more recent studies demonstrated heterogeneity in insulin secretion in dissociated mouse or human β-cells using the hemolytic plaque assay (2). Patch-clamp measurements also revealed heterogeneity in dissociated β-cell electrical properties (3). Autofluorescence measurements revealed heterogeneity in redox state, and incorporation of radioactive tracers revealed heterogeneity in glucose metabolism and insulin biosynthesis (4). The development of fluorescent biosensors and confocal or 2-photon microscopy provided tools to further characterize β-cell functional differences. This includes precise quantification of heterogeneity in dissociated β-cell glucose metabolism and redox state (5); glucose sensitivity to Ca2+ elevations and Ca2+ oscillation patterns (6); and cAMP oscillation patterns (7). Recently, the application of new biomarkers or high-throughput single-cell analyses has further revealed molecular details underlying β-cell heterogeneity.
Markers of β-Cell Subpopulations
Early studies suggested insulin granularity was a morphological marker that could separate a population of β-cells with a low glucose threshold (4). More recently, several markers have been used that reveal β-cell subpopulations with differing function. Polysialylated-neural cell adhesion molecule (PSA-NCAM) separated two populations of mouse β-cells, with one population (βhigh) showing higher Ca2+ and ATP elevation, insulin secretion, and Gck and Glut2 expression (8). Insulin promoter activity (MIP-GFP fluorescence) separated three populations of β-cells, with the MIP-GFPlow population (∼10% incidence in adult) possessing low insulin expression and low granularity (9). Aguayo-Mazzucato et al. (10) subsequently showed that the MIP-GFPlow and MIP-GFPhigh populations decreased and increased in incidence, respectively, during aging. Further, the MIP-GFPlow population was not marked by increases in the aging markers IGF1R or p16Ink4a, the lack thereof indicative of newly formed cells. The authors also showed that a population of highly secreting cells is lost during aging. However, the low incidence of this population (∼5%) is suggestive of a subset of MIP-GFPhigh cells. Recently, Lickert and colleagues (11) used a Fltp-Venus reporter mouse to separate two populations of β-cells. Fltp-Venus positive (Fltp+) cells showed improved insulin secretion but reduced proliferative ability, together with more extensive mitochondrial morphology, more oxidative metabolism, and increased insulin granularity. The genetic profile of Fltp+ cells was also consistent with improved function (i.e., increased expression of genes for GCK, GLUT2, GLP1R, GIPR, and Cx36), as well as increased maturity. Notably, during development or upon metabolic stress the Fltp− population showed greater proliferation and number. These studies together indicate a dynamic β-cell subpopulation defined by improved glucose-stimulated insulin secretion as a result of elevated glucose metabolism and insulin granule content.
Huising and colleagues (12) used an Ucn3 reporter mouse to reveal a population of insulin+ Ucn3− cells undergoing α-cell–to–β-cell transition at the intact islet periphery. These immature cells showed decreased expression of genes regulating glucose metabolism (Gck, tricarboxylic acid cycle, oxidative phosphorylation), membrane potential (KATP, CaV subunits), and Glp1r and Ins. They also lacked both glucose influx and glucose-regulated membrane potential and Ca2+ influx. Although reminiscent of subpopulations with reduced function, this population is much lower in incidence (∼2%), shows negligible glucose sensitivity, and is not differentiated by markers such as Fltp. As such, this represents a novel cell subpopulation. Herold and colleagues (13) also identified a population of β-cells in NOD mice characterized by reduced granularity that increased in number during autoimmune attack of the islet. This population also displayed reduced levels of insulin, GLUT2, and maturity markers, consistent with earlier studies (1). Thus, rarer subpopulations of β-cells do exist, although how they overlap is not known.
In human β-cells, Grompe and coworkers (14) identified two surface markers (CD9, ST8SIA1) that separated four populations of β-cells with distinct gene expression profiles. ST8SIA1+ cells (∼15% in healthy donors) showed lower insulin secretion and lower expression of GLUT2 but increased expression of genes encoding KATP channel subunits and other K+ channels. The incidence of ST8SIA1+ cells is also increased in donors with type 2 diabetes.
Although these markers all separate β-cell populations with distinct functional states, further less well-characterized subpopulations have been identified using other markers (see Roscioni et al. [15]). However, it is important to remember that markers are not generally associated with driving the functional state of the β-cell subpopulation. For example, a Fltp deletion has minor impact on β-cell function (11). Thus, PSA-NCAM or CD9/ST8SIA1 may simply separate differing populations in an arbitrary manner. Furthermore, it is not always clear whether fluorescent reporter constructs reflect the protein level. For example, MIP-GFPmedium and MIP-GFPhigh β-cells show identical insulin transcription and protein levels (9), and the change in Fltp gene transcription only varies ∼twofold between Fltp+ and Fltp− cells defined by the Fltp-Venus reporter (11). Thus, great care should be taken when linking the marker involved to the characteristics of the subpopulation in question.
High-Throughput Single-Cell Analysis
In an elegant study, Kaestner and colleagues (16) used mass cytometry to detect large numbers of protein markers in human β-cells, indicating three consistent β-cell states (C1, C2, C3). One state (C1, 10–70% in adults) showed substantially lower proliferation than the other two states (C2, C3), where the proportion of C1 increased with age and decreased in obesity. Although limited probes for proteins involved in β-cell function were used, the C1 population showed higher PDX1 and insulin expression indicative of a mature functional population. The C1 population also showed lower ST8SIA1 and CD9 levels compared with proliferating C2, C3 populations, where previously ST8SIA1− and CD9− β-cells showed improved insulin secretion and maturity markers and decreased in proportion during type 2 diabetes (14).
Single-cell sequencing has been used by several groups to examine the transcriptional profile in islet endocrine cells (17,18) (for a comprehensive review on this topic, see Gutierrez et al. [19]). This has also resolved subpopulations of β-cells in humans (17), identifying five β-cell states. Interestingly, all showed similar insulin expression but differed in gene transcription that included free fatty acid receptor 4 and RBP4 (an adipokine receptor). However, it remains unclear whether these states overlap with those revealed by mass cytometry or markers described to date. Further, no clear heterogeneity was detectable using similar analyses in mouse β-cells (20), questioning whether this may reflect true species differences, a reduction in variability due to use of syngeneic donors maintained in controlled housing conditions, or an effect of isolation protocol. Moreover, single-cell analysis characterizes heterogeneity in dissociated β-cells, and islet dissociation may bias the population of cells that are analyzed, for example, due to reduced β-cell viability. Integration of multiple factors (e.g., environmental stimuli, developmental cues, cell cycle and tissue architecture) that affect cell identity will also be important in future studies to robustly define subpopulations (21).
Interpreting Subpopulations From Marker and Single-Cell Analyses
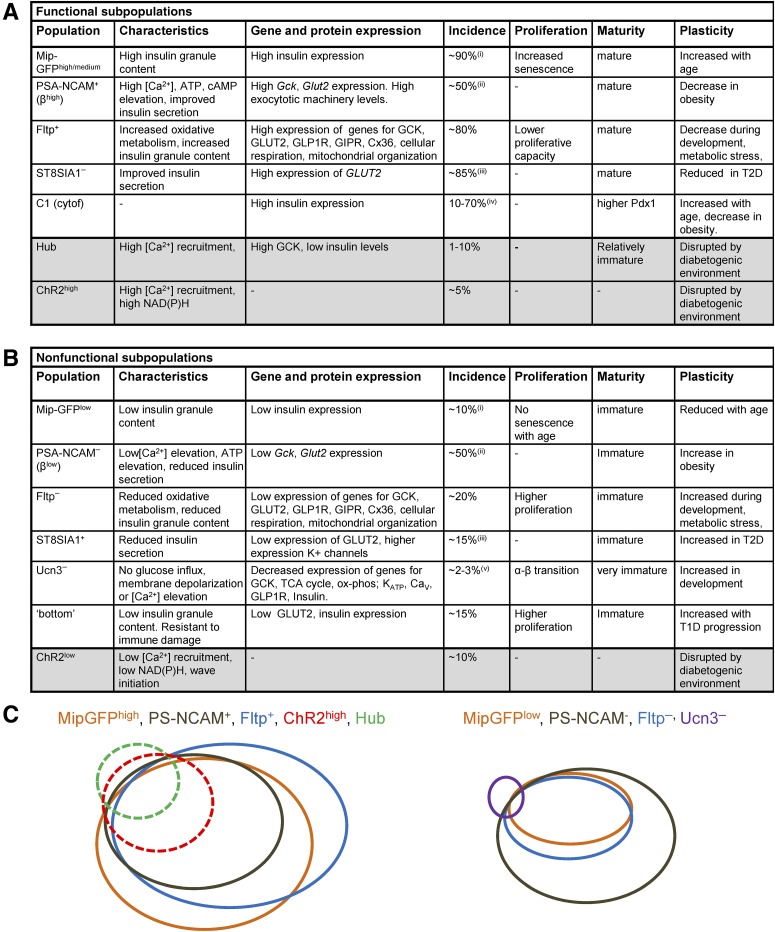
Figure 1 summarizes the functional β-cell subpopulations identified by recent marker and single-cell sequencing studies and their relative overlap in characteristics. Bearing in mind potential limitations in marker studies or high-throughput single-cell studies, these studies consistently show a mature population of β-cells (incidence 50–80%) that is functionally competent, probably due to higher rates of oxidative metabolism and ATP production; high levels of insulin expression, granule content, and secretion; and potentially improved electrical properties. This population reduces in incidence or shows dysfunction under aging and diabetes, possibly due to increased susceptibility to endoplasmic reticulum stress (22). Conversely, the opposing less mature population (incidence 15–50%) shows reduced insulin secretion secondary to reductions in glucose metabolism and insulin expression but has improved proliferative capacity.
Figure 1.
Summary of previously identified β-cell subpopulations using biomarker or single-cell analyses. A: Summary of functionally competent (functional) cell subpopulations, including their functional and gene/protein expression profile, incidence, and how they change in conditions associated with diabetes. B: Summary of less functional (nonfunctional) cell subpopulations. For A and B, shaded rows are functionally defined via optogenetics in situ. C: Schematic suggesting qualitative overlap between several identified subpopulations. Dashed lines indicate those defined via optogenetics in situ. Note the strong overlap between most identified larger populations, but the low-incidence functional hub cell subpopulation and nonfunctional Ucn3− subpopulation show relatively lower overlap. This may reflect the number and nature of the markers measured, as well as the functional readout. ox-phos, oxidative phosphorylation; T1D, type 1 diabetes; TCA, tricarboxylic acid. (i) GFPhigh increases from 10–40% with age (from 5–9 weeks to 16–40 weeks) and GFPlow decreases from 50 to 10% with age. (ii) PSA-NCAM+ is defined as top 50% expression. (iii) Incidence was 50% in donors with type 2 diabetes (T2D). (iv) Incidence was <30% in donors with T2D. (v) Ucn3−, insulin+ is located at the islet periphery. Adapted from Servier Medical Art under a CC-BY3.0 license (https://creativecommons.org/licenses/by/3.0/).
Although some studies indicate that more than two populations exist (e.g., MIP-GFPlow/medium/high, CD9+/−/ST8SIA1+/−), it is unclear whether the “functionally competent” and “less functional” populations can be further subdivided. Likely, there exist small distinct subpopulations, such as the Ucn3−insulin+ neogenic niche, with some similarities to the less functional population. Future work will require caution when determining whether identified subpopulations truly are distinct or whether they are an artifact of the analysis used. For example, does increasing the number of markers generate more or less apparent/overlapping β-cell populations, or can populations be reproduced in a second data set (e.g., using training and test sets)? Can the absence of a subpopulation be excluded given the relatively poor resolution of 'omics approaches such as RNA-Seq, where even at 6,000 M reads a proportion of genes remains unquantified?
Importantly, most studies to date described above have characterized subpopulations using single-cell preparations that lack the influence of the interactions between β-cells and other cell types within the islet. Although reaggregation methods can recover some of these influences, it is still unclear how different subpopulations may interact within the islet to influence islet function. Therefore, proper consideration of how a heterogeneous population of β-cells functions within the islet is needed.
How Heterogeneous Cells Interact in the Islet
Interactions between cells within the islet are critical: dissociation of the islet into single β-cells reduces the dynamic range of glucose-stimulated insulin secretion by ∼10-fold (23). Even when β-cells remain coupled to one or two other β- or α-cells, insulin secretion significantly improves (24). Although many mechanisms of cell–cell communication have been identified, less is known about how they coordinate heterogeneous cell populations.
Gap Junction Electrical Coupling Between Heterogeneous Cells
Gap junction (GJ) channels electrically couple β-cells within mouse and human islets (25), serving two main functions. First, GJ channels coordinate oscillatory dynamics in electrical activity and Ca2+ under elevated glucose or GLP-1, allowing pulsatile insulin secretion (26,27). Second, GJ channels lower spontaneous elevations in Ca2+ under low glucose levels (28). GJ coupling is also heterogeneous within the islet (29), leading to some β-cells being highly coupled and others showing negligible coupling.
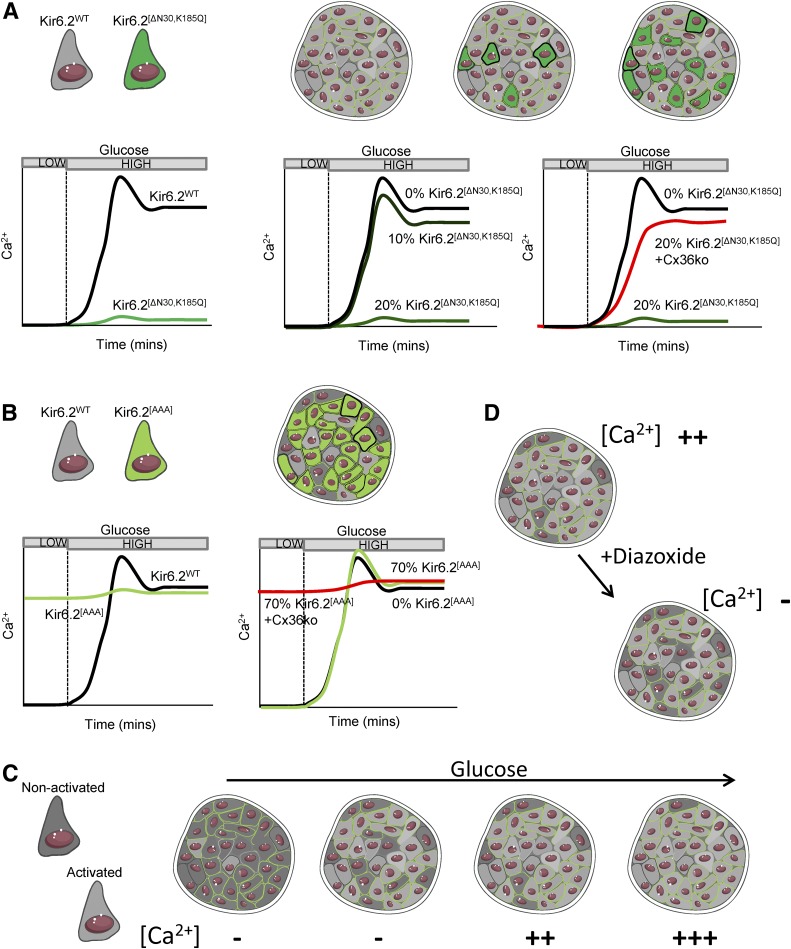
Several studies have examined how electrically heterogeneous cells interact via GJ channels using KATP channel mutant mice (Fig. 2). The Kir6.2[ΔN30,K185Q] mouse model CreER-dependently expresses overactive KATP channels in a population of β-cells, rendering them inexcitable (30). By varying recombination efficiency, as few as ∼15% inexcitable β-cells were found to be sufficient to suppress Ca2+ responses to glucose across the islet (30). Thus, small numbers of inexcitable cells can cause marked dysfunction by suppressing islet electrical activity and insulin secretion. Similar conclusions were made using the Kir6.2[AAA] mouse model that expresses inactive KATP channels in ∼70% of β-cells, rendering them hyperexcitable (31). Despite the majority of β-cells lacking glucose-regulated KATP closure, islets from these mice showed near-normal responses, as the remaining ∼30% of normal β-cells were sufficient to regulate islet Ca2+ (31). In each case, a deficiency in GJ channels led to KATP mutant cells and normal cells behaving distinctly (30,31). Thus, GJ coupling is a major mechanism by which electrically heterogeneous cells interact. Early studies imposing metabolic heterogeneity through a knockout of GCK in a population of β-cells did not find evidence of significant metabolic coupling between β-cells (5).
Figure 2.
Example for how heterogeneous cells can interact. A: Introducing Kir6.2[ΔN30,K185Q] into β-cells renders them unresponsive to glucose. With a small (∼10%) population of these less functional cells in the islet (dark green), glucose responsiveness is unchanged. However, with ∼20% of these cells, glucose responsiveness is lost. Glucose responsiveness is recovered by electrically isolating (Cx36ko) these less functional cells. B: Conversely, if a large proportion (∼70%) of hyperexcitable Kir6.2[AAA] cells are introduced into the islet (light green), glucose responsiveness remains, although this is lost when electrical coupling is lost (Cx36ko). C: In the native islet, the number of intrinsically activated (light gray) cells increases as glucose concentration rises. However, only when a certain threshold of cells are activated does Ca2+ elevate as a result of insufficient numbers of nonactivated cells (dark gray), analogous to A and B. D: The presence of activated and nonactivated cells can be further revealed by the KATP opener diazoxide, which renders only a small number of cells intrinsically inactive as observed in the absence of GJ coupling but fully abolishes Ca2+ elevations in the presence of GJ coupling. Adapted from Servier Medical Art under a CC-BY3.0 license (https://creativecommons.org/licenses/by/3.0/).
This series of experiments indicate a “bistability” in islet function, where a threshold number of poorly responsive β-cells is sufficient to totally suppress islet function. Notably, when islets lacking GJ channels are treated with low levels of the KATP activator diazoxide or the GCK inhibitor mannoheptulose, a subpopulation of cells are silenced, presumably corresponding to the less functional population (30). Only diazoxide/mannoheptulose concentrations capable of silencing >40% of these cells will fully suppress Ca2+ elevations in normal islets. Again, this indicates that a threshold number of poorly responsive cells can inhibit the whole islet. Thus, if there exists a threshold number of functionally competent β-cells (∼60–85%), then the islet will show coordinated elevations in Ca2+ and insulin secretion.
Below this threshold number, the islet will lack Ca2+ elevation and insulin secretion (Fig. 2). The precise threshold depends on the characteristics of the excitable and inexcitable populations: small numbers of inexcitable cells will increase the number of functionally competent cells required for islet activity, whereas small numbers of highly excitable cells will do the opposite. However, if GJ coupling is lowered, then inexcitable cells will exert a reduced suppression, also decreasing the threshold required.
Paracrine and Juxtacrine Communication
Paracrine communication between β-cells and other endocrine cells is also important for regulating insulin secretion. Complex cross talk between α- and β-cells—stemming from neurotransmitters, ions, and hormones—shapes glucagon and insulin secretion depending on glucose concentration (reviewed in Gaisano et al. [32]). δ-Cell somatostatin secretion also inhibits insulin secretion. Notably, Ucn3 cosecreted with insulin promotes somatostatin secretion, leading to negative feedback against overt fluctuations in insulin secretion (33). Therefore, dynamic intercommunication exists between the main endocrine cells types within the islet. Several neurotransmitters (e.g., acetylcholine, glutamate, GABA) are also released within the islet by nerve terminals and endocrine cells, which can modulate insulin secretion and act on other endocrine cells (reviewed in Caicedo [34]).
Juxtacrine- or contact-dependent communication includes EphA-ephrinA bidirectional signaling between β-cells (35). EphA forward signaling predominates at elevated glucose and promotes insulin secretion, but ephrinA reverse signaling predominates at low glucose and suppresses insulin secretion (35). NCAM signaling between β-cells also promotes insulin secretion (8).
Little is known how these paracrine and juxtacrine mechanisms impact heterogeneous cells. One possibility is a homogenizing effect. For example, given heterogeneity, some β-cells will secrete more insulin and cosecrete more Ucn3, thus stimulating more local somatostatin secretion. As a result, there may be a greater local suppression of insulin secretion, creating more homogeneous release. Whether such a homogenizing effect occurs with the various paracrine communication feedback loops (e.g., somatostain–Ucn3, glucagon–insulin) remains to be determined. Similarly, juxtacrine mechanisms may impact heterogeneous cells differently, particularly give heterogeneity in β-cell glucose metabolism. However, these actions also remain to be determined.
Heterogeneity in the Intact Islet
Functional heterogeneity in the islet in terms of glucose metabolism (NADH response, GCK expression) (5,36) has been long known. Although electrical activity and cAMP responses are apparently homogeneous in the islet (26,37), this is likely due to GJ coupling. In the absence of GJ coupling, the electrical response is heterogeneous in terms of glucose sensitivities and patterns of Ca2+ oscillations (26). Despite several aspects of function being homogeneous in the islet, β-cell subpopulations can dramatically influence overall islet function as discussed above.
A Role for Existing Subpopulations?
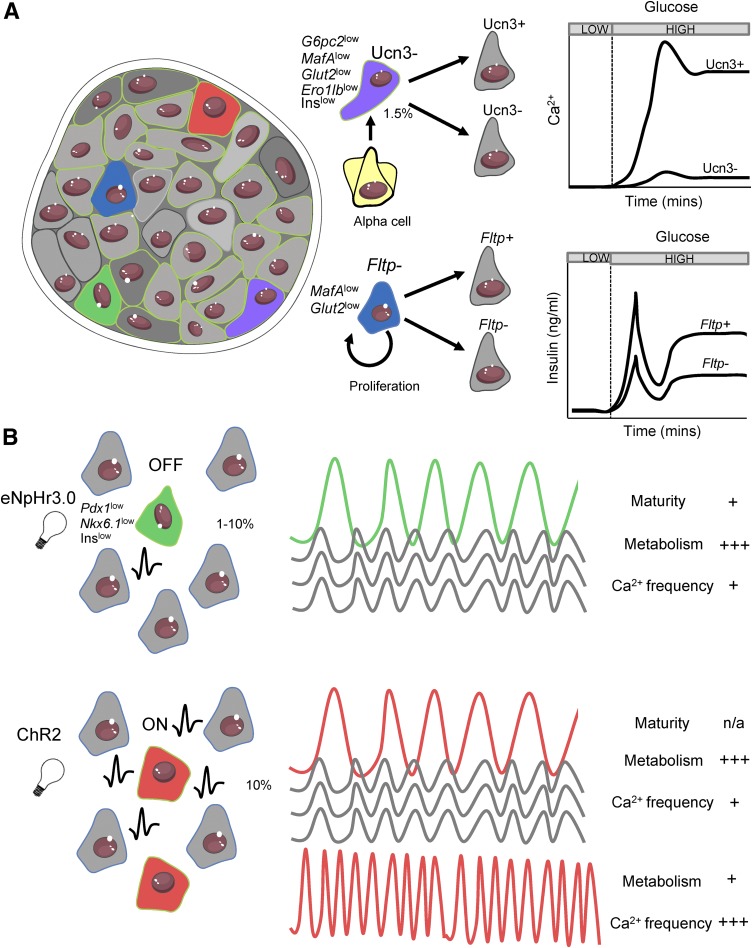
MIP-GFPhigh, PSA-NCAMhigh, Fltp+, and ST8SIA1− functionally competent β-cell subpopulations all show similar characteristics with incidences of 50–80%. Given our understanding of how heterogeneous cells interact via GJ coupling, it is tempting to speculate that the opposing less functional β-cell subpopulation (i.e., MIP-GFPlow, PSA-NCAMlow, Fltp−, ST8SIA1+; 15–50%) could significantly suppress islet activity via electrical coupling. However, Fltp− cells showed a marked reduction in Gjd2 expression encoding Cx36, which would be expected to reduce their ability to suppress Ca2+ elevations across the islet, thus allowing for normal islet function. As such, the increased number of Fltp− cells in development or metabolic stress will likely not impair islet function. Whether MIP-GFPlow, PSA-NCAMlow, or ST8SIA1+ cells show reduced Gjd2/Cx36 expression remains to be determined. Therefore, as a result of functional remodeling, the smaller less functional immature β-cell population within the islet is unlikely to have a detrimental effect on overall islet function. Until recently it has been unclear whether further subdivisions within the functionally competent subpopulation have a disproportionate role within the islet, in part owing to a lack of means to discover these subdivisions.
Optogenetics
Interrogation of β-cell function within islets requires technologies with high spatiotemporal resolution to capture and manipulate signaling events that occur throughout the islet. This can be achieved using optogenetics, where ion channels or pumps derived from light-sensitive bacteria are recombinantly expressed in the cell membrane to optically modulate electrical excitability and β-cell function (38). Combined with high-speed optical imaging, this provides a powerful platform for understanding subpopulation function. Pioneering studies by Reinbothe et al. (38) showed the utility of channelrhodopsin-2 (ChR2), a light-activated cation channel, to modulate islet Ca2+ fluxes using blue light.
Later studies used halorhodopsin (eNpHR3.0), an orange light–activated Cl− pump, and ChR2 to selectively silence or activate single β-cells, as well as clusters and larger regions (39,40) (Fig. 3).
Figure 3.
Heterogeneity in the intact islet. A: Different β-cell subpopulations coexist within the islet, each characterized by different gene/protein expression patterns, morphological markers, glucose responsiveness, insulin secretion, and proliferative capacity and function (Fig. 1). Fltp− proliferative (blue) and Ucn3− transdifferentiating (purple) less functional immature populations are likely nonelectrically coupled and thus do not significantly affect islet-wide responses to glucose. B: Optogenetic mapping reveals highly functional β-cell subpopulations with varying identity markers, metabolic properties, and Ca2+ responses, but with the ability to exert disproportionate control over coordination and intraislet Ca2+ responses. This includes eNpHR3.0-silenced β-cells (hub cells, green) in which halorhodopsin activation and membrane hyperpolarization disproportionally silence the islet and ChR2-activated cells (red) in which ChR2 activation and membrane depolarization disproportionally activate the islet. A population of cells in which ChR2 activation has little effect also shows pacemaker-like characteristics owing to their higher intrinsic oscillation frequency. Adapted from Servier Medical Art under a CC-BY3.0 license (https://creativecommons.org/licenses/by/3.0/).
Using Optogenetics to Discover and Understand β-Cell Subpopulations
Optogenetic mapping has provided evidence that a small subset of functionally competent β-cells may play a disproportionate role in orchestrating islet responses to glucose (Fig. 3) (39). Random activation of single β-cells using ChR2 revealed significant elevation of [Ca2+]i in neighboring regions in ∼50% of cases, presumably corresponding to the functionally competent β-cell subpopulation. However, substantial activation was achieved in rare cases (∼5%), with Ca2+ spreading throughout large portions of the islet. Supporting an important link between glucose metabolism and heterogeneity indicated in prior studies, elevation of Ca2+ was most effective in stimulated cells that had the highest NAD(P)H responses (40). Furthermore, a separate subregion of β-cells was identified, characterized by low NAD(P)H responses, that showed high intrinsic oscillatory frequencies and corresponded to initiating Ca2+ wave propagation (40). Along similar lines, targeted silencing using eNpHR3.0 highlighted a subpopulation (∼1–10%) of metabolically adapted β-cells involved in supporting islet-wide Ca2+ dynamics (39). These cells, termed hub cells, were typified by elevated GCK content and hyperpolarized mitochondria, highly consistent with other functionally competent subpopulations. However, they also possessed ∼two- to fivefold lower protein expression levels of Pdx1/Nkx-6.1/insulin, indicating relative immaturity, although all three markers were still detectable (Figs. 1 and 3). Whether eNpHR3.0-defined hub cells and ChR2-defined subpopulations are fixed in time or dynamic remains unknown due to the inability to record for more than a few hours, although longitudinal intravital imaging techniques may aid this (41). That these more distinct populations were missed in biomarker analysis or high-throughput single-cell analysis likely indicates the importance of their presence in the intact islet for function and further argues for the importance of studying β-cell heterogeneity in the islet context.
An improvement in metabolic properties and function in hub cells is difficult to reconcile with a loss of β-cell identity. However, it is worth noting that glucose-stimulated insulin secretion in MafA-deficient β-cells (i.e., less mature) is only reduced by ∼50% with minimally affected NAD(P)H responses (42), and islets deficient in GLUT2 display preserved second-phase insulin secretion (43). Moreover, recent studies have shown that immature, proliferative β-cells highly express genes involved in amino acid metabolism and mitochondrial function (44). Last, subpopulations of mouse β-cells exist with high Gck but low Pdx1/Nkx-6.1 (20) and low insulin expression (45). Thus, these data indicate a subdivision within the functional population in the islet, raising interesting questions about the role of β-cell identity in islet function and insulin secretion.
Extrapolating Single-Cell Analyses to Islet Studies
Some caution is required when trying to extrapolate results between transcriptional and functional studies. As discussed above, it is well acknowledged that tissue architecture influences gene expression and dissociated cell mRNA profiles may not necessarily reflect those in situ (46). Isolation of single cells is usually associated with decreases in viability. Although these cells are excluded from RNA-Seq analyses of purified populations, some subpopulations, including hub cells (39) or those that express low SERCA2/low Pdx1 (47), appear more susceptible to endoplasmic reticulum stress and apoptosis/cell death. As such, their gene/protein expression levels may not be fully captured. Last, there is a tendency to classify and label subpopulations, whereas β-cells likely occupy an overlapping continuum due to the dynamic nature of gene transcription (46). Thus, a major obstacle to our present understanding of heterogeneity is the inability to track transcriptional/protein dynamics in single β-cells in situ and over long time scales.
Islet Heterogeneity In Vivo
Islets are highly vascularized, and pioneering intravital imaging studies in mice have shown that islet blood flow is directional, perfusing α-cells before β-cells (48). Key to understanding heterogeneity in vivo will be experiments using islets transplanted into the anterior chamber of the eye (41), where blood and neural supplies remain intact. Combined with optogenetics and reporter gene imaging (see below), this provides a powerful means to longitudinally image the plasticity of subpopulations across the life span. However, the anterior chamber of the eye is unable to recapitulate normal pancreatic physiology (e.g., endocrine–exocrine interactions), and immune infiltration has been reported (49).
Future Tools for the Interrogation of β-Cell Heterogeneity
We have argued the importance for resolving and studying β-cell heterogeneity in the context of the intact islet. Although we have discussed several new technologies that have been developed and applied to examine β-cell heterogeneity, additional technologies show great promise for studying β-cell heterogeneity in situ.
Photopharmacology
Photopharmacology describes the synthesis of pharmacophores bearing azobenzene photoresponsive elements whose inactive and active states can be controlled by light through cis- and trans-isomerization. These approaches have been applied to antidiabetes agents including sulfonylureas or incretin mimetics (50). A major advantage of photopharmacology over optogenetics is the ability to control endogenous targets without the need for recombinant approaches, thus providing high utility for targeting human β-cells. However, these approaches tend to be nonbinary, with some drug activity seen even with inactive or unilluminated compound, which can complicate functional interrogation of β-cell signaling.
Matrix-Assisted Laser Desorption/Ionization Imaging
Matrix-assisted laser desorption/ionization (MALDI) is a label-free method that provides information on endogenous proteins, metabolites, lipids, and hormones. This requires application of a matrix to allow absorption of laser energy followed by desorption and liberation of single protonated species, whose time-of-flight (TOF) is ranked according to analyte. Although the spatial resolution of MALDI-TOF is low (20–200 µm), many endogenous markers can be studied simultaneously in situ with high sensitivity. As such, it may be useful for understanding heterogeneity between individual islets or islet regions (51); however, the technique is temporally limited due to the requirement to use fixed tissue.
Multiplexed Ion Beam Imaging
Mass cytometry overcomes limitations in channel number with conventional flow cytometry by using rare earth–labeled antibodies and TOF detection. This enables large (>40) protein panels to be studied and has been applied to dissociated human β-cells, resolving three distinct subpopulations (16). Technology using rare-earth metal labeling and TOF detection has been adapted into an imaging format—multiplexed ion beam imaging (MIBI)—that can achieve subcellular spatial resolution (52). Therefore, imaging of islets in situ, including in intact pancreas preparations, will be achievable. One potential limitation will be the availability of compatible metal-labeled antibodies against proteins involved in β-cell function (e.g., GCK/ion channels/GPCRs).
Reporter Gene Imaging
Gene expression dynamics (46) may add another level of heterogeneity to β-cell function and potentially explain some of the differences in β-cell subpopulations observed to date. Early studies by Szabat et al. (45) used a Pdx1-mRFP/Ins1-GFP dual reporter to show the existence of a Pdx1+/Inslow subpopulation. A similar approach can be envisaged using a combination of fluorophores with long and short half-lives to allow measurement of protein expression dynamics (as an indirect readout for gene expression) in the intact islet setting and to examine plasticity in β-cell subpopulations. However, the disadvantage of such approaches is the inability to visualize transcription/translation of endogenous genes/RNAs or account for posttranscriptional regulation.
RNA Fluorescent In Situ Hybridization
Fluorescent in situ hybridization (FISH) allows nascent RNA to be labeled, providing a snapshot of transcription. High-throughput single-cell FISH has been applied to examine changes in mRNA levels for β-cell repressed genes during proliferation (53). Closer examination of the FISH data revealed marked differences between individual β-cells (10- to 100-fold), suggesting heterogeneity at the transcript level (53). By using higher resolution single-molecule detection (fliFISH) to robustly quantify RNA levels in pancreas tissue sections, marked heterogeneity in Ins2 and Nkx2–2 was observed between β-cells. This included observing more immature (low Ins2, Nkx2–2) signatures at the islet periphery (54).
CROP-Seq and Peturb-Seq
Differential gene expression can be obtained in single genome-edited cells residing within a pooled population by directly linking guide RNA expression with individual cell transcriptome responses, for example, via incorporation of polyadenylated sequences recognized by RNA-Seq or combining gRNA with expression of a bar code (55). Both techniques allow high-throughput CRISPR screening of signaling/regulatory pathways in heterogeneous populations and could potentially be used to understand the interactions underlying heterogeneity at different developmental stages.
Computer Modeling
To move beyond a qualitative overview (Fig. 1), computer models show promise in reconciling divergent data sets generated from the range of available technologies ('omics, imaging) and sample preparations (mouse, human, intact, dissociated). Computer models accurately described how a defined β-cell population impacts islet stimulus secretion, e.g., KATP channel mutant (30). Computer models also accurately described stimulus secretion in subpopulations uncovered through ChR2 stimulation (40). We envision that islets could be modeled to incorporate subpopulations of β-cells with characteristics defined by single-cell approaches, and these models could be compared with functional studies. Rapid in silico screening could then identify overlap between subpopulations identified using different experimental approaches and predict how they would influence islet function. A necessary step will be validating theoretical predictions with focused experiments.
Summary
Spurred on by technological advances in single-cell genomics and imaging, the renewed interest in β-cell heterogeneity has changed our view of islet development and function. Pertinently, β-cell subpopulations have been identified that provide deeper mechanistic understanding of poorly characterized processes, such as proliferation, differentiation/maturation, and stimulus secretion. Thus, β-cell heterogeneity has highlighted new facets of islet plasticity and how changes in this may affect islet function under conditions associated with diabetes. However, most studies to date have investigated dissociated cells, where many transcriptomic/protein features may be altered. Moreover, although the molecular signatures of subpopulations are well characterized, how these influence protein expression and functional state are poorly understood. Key to better understanding heterogeneity will be studies in the intact tissue and even in vivo, the integration of multiple facets of a cell’s identity, and models with better fidelity for reading out gene and protein expression dynamics. Indeed, several gaps in our knowledge remain: how do we robustly define a β-cell subpopulation? What is the molecular basis and functional role for heterogeneity among other endocrine cells (α-cells, δ-cells)? Are subpopulations of β-cells (or other cells) fixed into a specific functional state, or are they transitional? Recent and future technological developments should allow these questions to be addressed and, in doing so, may reveal new genes and pathways that can be harnessed to improve therapy, coax β-cell regeneration, and inform de novo engineering of islets and islet transplantation.
Article Information
Funding. R.K.P.B. was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants R01 DK102950 and R01 DK106412, National Institutes of Health Office of the Director grant OT2 OD023852, and JDRF grants 5-CDA-2014-198-A-N and 1-INO-2017-435-A-N. D.J.H. was supported by a Diabetes UK R.D. Lawrence Fellowship (12/0004431), a Diabetes UK research grant (17/0005681), a Wellcome Trust Institutional Support Award, and a Medical Research Council project grant (MR/N00275X/1). This work has received funding from the Horizon 2020 European Research Council research and innovation programme (starting grant 715884 to D.J.H.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
References
- 1.Pipeleers DG. Heterogeneity in pancreatic beta-cell population. Diabetes 1992;41:777–781 [DOI] [PubMed] [Google Scholar]
- 2.Salomon D, Meda P. Heterogeneity and contact-dependent regulation of hormone secretion by individual B cells. Exp Cell Res 1986;162:507–520 [DOI] [PubMed] [Google Scholar]
- 3.Misler S, Falke LC, Gillis K, McDaniel ML. A metabolite-regulated potassium channel in rat pancreatic B cells. Proc Natl Acad Sci U S A 1986;83:7119–7123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiekens R, In ’t Veld P, Mahler T, Schuit F, Van De Winkel M, Pipeleers D. Differences in glucose recognition by individual rat pancreatic B cells are associated with intercellular differences in glucose-induced biosynthetic activity. J Clin Invest 1992;89:117–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piston DW, Knobel SM, Postic C, Shelton KD, Magnuson MA. Adenovirus-mediated knockout of a conditional glucokinase gene in isolated pancreatic islets reveals an essential role for proximal metabolic coupling events in glucose-stimulated insulin secretion. J Biol Chem 1999;274:1000–1004 [DOI] [PubMed] [Google Scholar]
- 6.Zhang M, Goforth P, Bertram R, Sherman A, Satin L. The Ca2+ dynamics of isolated mouse beta-cells and islets: implications for mathematical models. Biophys J 2003;84:2852–2870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dyachok O, Isakov Y, Sågetorp J, Tengholm A. Oscillations of cyclic AMP in hormone-stimulated insulin-secreting beta-cells. Nature 2006;439:349–352 [DOI] [PubMed] [Google Scholar]
- 8.Karaca M, Castel J, Tourrel-Cuzin C, et al. . Exploring functional β-cell heterogeneity in vivo using PSA-NCAM as a specific marker. PLoS One 2009;4:e5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katsuta H, Aguayo-Mazzucato C, Katsuta R, et al. . Subpopulations of GFP-marked mouse pancreatic β-cells differ in size, granularity, and insulin secretion. Endocrinology 2012;153:5180–5187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguayo-Mazzucato C, van Haaren M, Mruk M, et al. . β cell aging markers have heterogeneous distribution and are induced by insulin resistance. Cell Metab 2017;25:898–910.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bader E, Migliorini A, Gegg M, et al. . Identification of proliferative and mature β-cells in the islets of Langerhans. Nature 2016;535:430–434 [DOI] [PubMed] [Google Scholar]
- 12.van der Meulen T, Mawla AM, DiGruccio MR, et al. Virgin beta cells persist throughout life at a neogenic niche within pancreatic islets. Cell Metab 2017;25:911–926.e6 [DOI] [PMC free article] [PubMed]
- 13.Rui J, Deng S, Arazi A, Perdigoto AL, Liu Z, Herold KC. β Cells that resist immunological attack develop during progression of autoimmune diabetes in NOD mice. Cell Metab 2017;25:727–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorrell C, Schug J, Canaday PS, et al. . Human islets contain four distinct subtypes of β cells. Nat Commun 2016;7:11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roscioni SS, Migliorini A, Gegg M, Lickert H. Impact of islet architecture on β-cell heterogeneity, plasticity and function. Nat Rev Endocrinol 2016;12:695–709 [DOI] [PubMed] [Google Scholar]
- 16.Wang YJ, Golson ML, Schug J, et al. . Single-cell mass cytometry analysis of the human endocrine pancreas. Cell Metab 2016;24:616–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segerstolpe Å, Palasantza A, Eliasson P, et al. . Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab 2016;24:593–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xin Y, Kim J, Okamoto H, et al. . RNA sequencing of single human islet cells reveals type 2 diabetes genes. Cell Metab 2016;24:608–615 [DOI] [PubMed] [Google Scholar]
- 19.Gutierrez GD, Gromada J, Sussel L. Heterogeneity of the pancreatic beta cell. Front Genet 2017;8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xin Y, Kim J, Ni M, et al. . Use of the fluidigm C1 platform for RNA sequencing of single mouse pancreatic islet cells. Proc Natl Acad Sci U S A 2016;113:3293–3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner A, Regev A, Yosef N. Revealing the vectors of cellular identity with single-cell genomics. Nat Biotechnol 2016;34:1145–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szabat M, Page MM, Panzhinskiy E, et al. . Reduced insulin production relieves endoplasmic reticulum stress and induces β cell proliferation. Cell Metab 2016;23:179–193 [DOI] [PubMed] [Google Scholar]
- 23.Lernmark A. The preparation of, and studies on, free cell suspensions from mouse pancreatic islets. Diabetologia 1974;10:431–438 [DOI] [PubMed] [Google Scholar]
- 24.Wojtusciszyn A, Armanet M, Morel P, Berney T, Bosco D. Insulin secretion from human beta cells is heterogeneous and dependent on cell-to-cell contacts. Diabetologia 2008;51:1843–1852 [DOI] [PubMed] [Google Scholar]
- 25.Cigliola V, Chellakudam V, Arabieter W, Meda P. Connexins and β-cell functions. Diabetes Res Clin Pract 2013;99:250–259 [DOI] [PubMed] [Google Scholar]
- 26.Benninger RK, Zhang M, Head WS, Satin LS, Piston DW. Gap junction coupling and calcium waves in the pancreatic islet. Biophys J 2008;95:5048–5061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodson DJ, Mitchell RK, Bellomo EA, et al. . Lipotoxicity disrupts incretin-regulated human β cell connectivity. J Clin Invest 2013;123:4182–4194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benninger RK, Head WS, Zhang M, Satin LS, Piston DW. Gap junctions and other mechanisms of cell-cell communication regulate basal insulin secretion in the pancreatic islet. J Physiol 2011;589:5453–5466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farnsworth NL, Hemmati A, Pozzoli M, Benninger RK. Fluorescence recovery after photobleaching reveals regulation and distribution of connexin36 gap junction coupling within mouse islets of Langerhans. J Physiol 2014;592:4431–4446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hraha TH, Westacott MJ, Pozzoli M, Notary AM, McClatchey PM, Benninger RK. Phase transitions in the multi-cellular regulatory behavior of pancreatic islet excitability. PLOS Comput Biol 2014;10:e1003819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rocheleau JV, Remedi MS, Granada B, et al. . Critical role of gap junction coupled KATP channel activity for regulated insulin secretion. PLoS Biol 2006;4:e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaisano HY, Macdonald PE, Vranic M. Glucagon secretion and signaling in the development of diabetes. Front Physiol 2012;3:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Meulen T, Donaldson CJ, Cáceres E, et al. . Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion. Nat Med 2015;21:769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caicedo A. Paracrine and autocrine interactions in the human islet: more than meets the eye. Semin Cell Dev Biol 2013;24:11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konstantinova I, Nikolova G, Ohara-Imaizumi M, et al. . EphA-Ephrin-A-mediated beta cell communication regulates insulin secretion from pancreatic islets. Cell 2007;129:359–370 [DOI] [PubMed] [Google Scholar]
- 36.Jetton TL, Magnuson MA. Heterogeneous expression of glucokinase among pancreatic beta cells. Proc Natl Acad Sci U S A 1992;89:2619–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian G, Sandler S, Gylfe E, Tengholm A. Glucose- and hormone-induced cAMP oscillations in α- and β-cells within intact pancreatic islets. Diabetes 2011;60:1535–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reinbothe TM, Safi F, Axelsson AS, Mollet IG, Rosengren AH. Optogenetic control of insulin secretion in intact pancreatic islets with β-cell-specific expression of Channelrhodopsin-2. Islets 2014;6:e28095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnston NR, Mitchell RK, Haythorne E, et al. . Beta cell hubs dictate pancreatic islet responses to glucose. Cell Metab 2016;24:389–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westacott MJ, Ludin NWF, Benninger RKP. Spatially organized β-cell subpopulations control electrical dynamics across islets of Langerhans. Biophys J 2017;113:1093–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Speier S, Nyqvist D, Cabrera O, et al. . Noninvasive in vivo imaging of pancreatic islet cell biology. Nat Med 2008;14:574–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hang Y, Yamamoto T, Benninger RKP, et al. . The MafA transcription factor becomes essential to islet β-cells soon after birth. Diabetes 2014;63:1994–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guillam M-T, Hümmler E, Schaerer E, et al. . Early diabetes and abnormal postnatal pancreatic islet development in mice lacking Glut-2. Nat Genet 1997;17:327–330 [DOI] [PubMed] [Google Scholar]
- 44.Zeng C, Mulas F, Sui Y, et al. . Pseudotemporal ordering of single cells reveals metabolic control of postnatal β cell proliferation. Cell Metab 2017;25:1160–1175.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szabat M, Luciani DS, Piret JM, Johnson JD. Maturation of adult beta-cells revealed using a Pdx1/insulin dual-reporter lentivirus. Endocrinology 2009;150:1627–1635 [DOI] [PubMed] [Google Scholar]
- 46.Harper CV, Featherstone K, Semprini S, et al. . Dynamic organisation of prolactin gene expression in living pituitary tissue. J Cell Sci 2010;123:424–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson JS, Kono T, Tong X, et al. . Pancreatic and duodenal homeobox protein 1 (Pdx-1) maintains endoplasmic reticulum calcium levels through transcriptional regulation of sarco-endoplasmic reticulum calcium ATPase 2b (SERCA2b) in the islet β cell. J Biol Chem 2014;289:32798–32810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nyman LR, Wells KS, Head WS, et al. . Real-time, multidimensional in vivo imaging used to investigate blood flow in mouse pancreatic islets. J Clin Invest 2008;118:3790–3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mojibian M, Harder B, Hurlburt A, Bruin JE, Asadi A, Kieffer TJ. Implanted islets in the anterior chamber of the eye are prone to autoimmune attack in a mouse model of diabetes. Diabetologia 2013;56:2213–2221 [DOI] [PubMed] [Google Scholar]
- 50.Broichhagen J, Frank JA, Trauner D. A roadmap to success in photopharmacology. Acc Chem Res 2015;48:1947–1960 [DOI] [PubMed] [Google Scholar]
- 51.Aichler M, Borgmann D, Krumsiek J, et al. . N-acyl Taurines and Acylcarnitines cause an imbalance in insulin synthesis and secretion provoking β cell dysfunction in type 2 diabetes. Cell Metab 2017;25:1334–1347.e4 [DOI] [PubMed] [Google Scholar]
- 52.Angelo M, Bendall SC, Finck R, et al. . Multiplexed ion beam imaging of human breast tumors. Nat Med 2014;20:436–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klochendler A, Caspi I, Corem N, et al. . The Genetic program of pancreatic β-cell replication in vivo. Diabetes 2016;65:2081–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cui Y, Hu D, Markillie LM, et al. . Fluctuation localization imaging-based fluorescence in situ hybridization (fliFISH) for accurate detection and counting of RNA copies in single cells. Nucleic Acids Res 2018;46:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wagner DE, Klein AM. Genetic screening enters the single-cell era. Nat Methods 2017;14:237–238 [DOI] [PubMed] [Google Scholar]