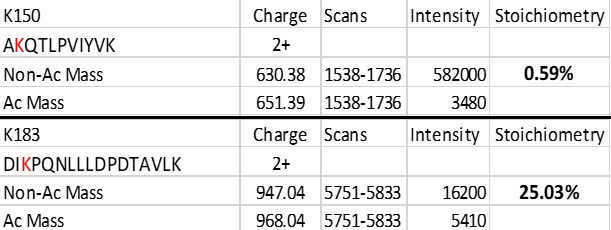
Figure 2. Molecular modeling and molecular dynamics simulations of GSK3β wild-type and K183 acetylated mutant.
(A) Annotation of representative tandem mass spectra of trypsin-digested GSK3β, depicting K150 and K183 acetylation. (B) Representation of the acetylation sites on the crystal structure of GSK3β (PDB ID 4NM0): (i) surface (ii) cartoon representation. (iii) Magnified active site representing position of K183 and (iv) magnified active site representing position of acetylated K183 (acK183). (C) Nucleotide-binding site in GSK3β crystal structure (PDB ID 4NM0) representing ADP, nucleotide interacting residues and K183. (D) Overlay of the wild-type (blue) and acK183 mutant (orange) of GSK3β representing the surface of ADP nucleotide, at random snapshots in the MD trajectory. (E) Overlay of protein backbone Cα RMSD plots of the five 20 ns MD trajectories in wild type. (F) Overlay of ADP nucleotide RMSD plots of the five 20 ns MD trajectories in wild type. (G) Overlay of the distance between the NZ atom of K85 and α-phosphate of ADP as a function of time for two stable trajectories (dark blue/cyan – wild type, pink/red – acK183). (H) Overlay of protein backbone Cα RMSD plots of the five 20 ns MD trajectories in acK183 mutant. (I) Overlay of ADP nucleotide RMSD plots of the five 20 ns MD trajectories in acK183 mutant. (J) Overlay of the distance between the NZ atom of K85 and β-phosphate of ADP as a function of time for two stable trajectories (dark blue/cyan – wild type, pink/red – acK183). (K) Histogram showing binding of γ−32P-ATP to recombinant wild type and mutants of His-GSK3β. Plasmids encoding wild type and mutants of His-GSK3β were transformed into E. coli BL21 (DE3). His-GSK3β and its mutants were purified by Ni-NTA affinity chromatography. n = 4 independent experiments. Data is presented as mean ± s.d. *p<0.05. One-way ANOVA was used to calculate the p values. (L) Histogram showing activity of HA-tagged WT or mutants of GSK3β. HA-tagged human GSK3β or its mutants were overexpressed in HeLa cells by transfection of their respective plasmids. HA-GSK3β or its mutants were immunoprecipitated using HA-coupled agarose beads (Sigma-Aldrich). The enzymatic activity of GSK3β was measured against glycogen synthase (GS)-peptide as described in the Materials and methods section. GSK3β-DN - GSK3β-K85A; Dominant negative. GSK3β-CA- GSK3β S9A; catalytically active. n = 4 independent experiments. Data is presented as mean ± s.d. *p<0.05. One-way ANOVA was used to calculate the p values.
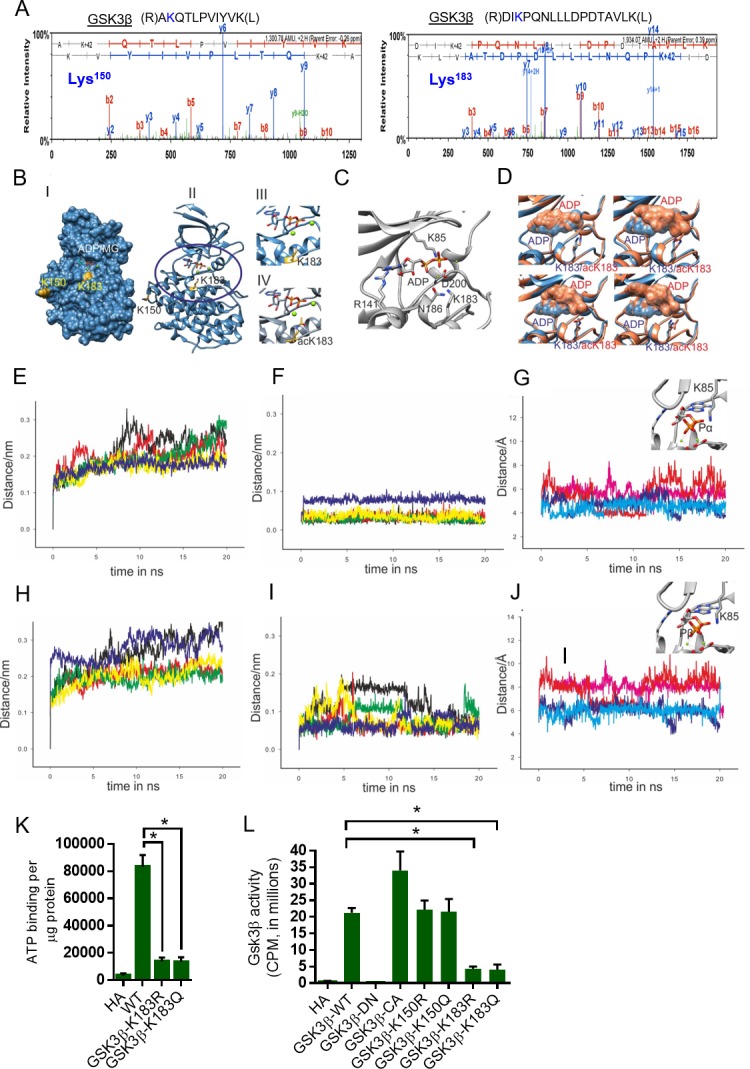
Figure 2—figure supplement 1. Protein backbone Cα RMSF plots of the wild type (dark blue) and Ac-K183 mutant (red).
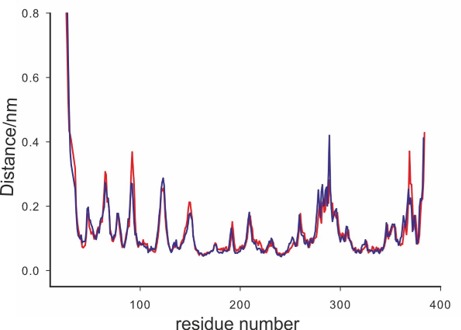
Figure 2—figure supplement 2. Homology alignment of GSK3β between different species.
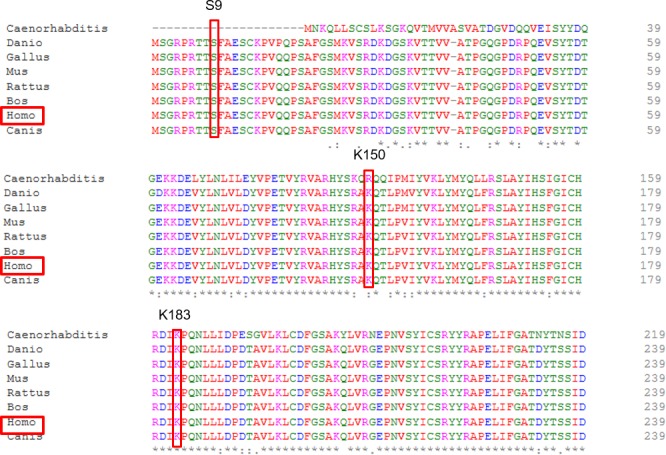
Figure 2—figure supplement 3. Stoichiometry for GSK3β-K150, -K183 acetylation.