Abstract
Background
Many extremely preterm infants have low vitamin D concentrations at birth, but early childhood outcomes after vitamin D supplementation have not been reported.
Objective
To determine a dose-response relationship between increasing doses of enteral vitamin D in the first 28 days after birth and cognitive scores at 2 years of age.
Methods
In this Phase II double-blind dose-response randomized trial, infants with gestational ages between 23 and 27 weeks were randomly assigned to receive placebo or a vitamin D dose of 200 IU/day or 800 IU/day from day 1 of enteral feeding to postnatal day 28. The primary outcome of this follow-up study was Bayley III cognitive score at 22 to 26 months of age.
Results
Seventy of 80 survivors had a follow-up evaluation at 2 years of age (88%). There were no significant differences in cognitive scores between supplementation groups (p=0.47). Cognitive scores did not differ between the higher vitamin D dose and placebo groups (median difference favoring the 800 IU group: +5 points; 95% CI −5 to 15; p=0.23). The linear trend between increasing doses of vitamin D and reduction of neurodevelopmental impairment (placebo group: 54%; 200 IU group: 43%; 800 IU group: 30%; p=0.08) or language impairment (placebo group: 64%; 200 IU group: 57%; 800 IU group: 45%; p=0.15) was not statistically significant. Respiratory outcomes at 2 years of age (need for supplemental oxygen or asthma medications) did not differ between groups.
Conclusion
In extremely preterm infants, early vitamin D supplementation did not significantly improve cognitive scores. Though underpowered for clinically meaningful differences in early childhood outcomes, this trial may help determine dosing for further investigation of vitamin D supplementation.
Keywords: Neurodevelopment, Neurocognitive outcomes, Respiratory outcomes, Nutritional requirements, Premature Infants
INTRODUCTION
Nearly 70% of extremely preterm infants (< 28 weeks of gestation) have low vitamin D concentrations at birth[1–3]. These infants depend on external sources of vitamin D[4,5] because the maternal-fetal transfer of vitamin D following preterm birth is abruptly terminated[5–8], their ability to synthesize vitamin D from natural ultraviolet light is limited[4,5,9], and the amount of vitamin D that they receive from human milk is low[4,9,10].
Many studies have shown that vitamin D modulates bone metabolism[11,12], a functionally measurable outcome of vitamin D deficiency, and is also associated with lung maturation[13], the immune response[14], and brain development[15,16]. In vitro, vitamin D signals neuronal differentiation and proliferation, regulates the metabolism of neurotrophic factors and neurotoxins, protects neurons during inflammation, and indirectly promotes brain growth through augmentation of endocrine functions[17,18].
Vitamin D supplementation in extremely preterm infants results in higher serum concentrations of vitamin D after supplementation[1–3,19]. However, the effects of vitamin D on neurodevelopmental and respiratory outcomes of this vulnerable population at 2 years of age have not been evaluated to date[12,18–20].
The specific aim of this follow-up study was to determine a dose-response effect between increasing doses of enteral vitamin D in the first 28 days after birth and cognitive scores at 22 to 26 months of age.
METHODS
In the initial phase II double-blind randomized controlled trial on early vitamin D supplementation, 100 extremely preterm infants with gestational ages of 23 weeks through 27 weeks were randomly assigned to receive either placebo, a vitamin D dose of 200 IU/day, or a vitamin D dose of 800 IU/day from day 1 of enteral feeding to postnatal day 28. The baseline estimated intake of vitamin D from parenteral and enteral nutrition was 200 IU/day in all study groups [5]. Written informed consent was obtained from each infant’s parent or guardian within 96 hours after birth. Research pharmacy staff not involved in patient care were responsible for performing block randomization using computerized random sequence generation, determining participant allocation to one of the supplementation groups by opening sequentially numbered sealed envelopes, and masking the individuals administering the assigned supplementation. Twins were randomized independently. The pharmacologic outcome of the primary study was vitamin D concentration at postnatal day 28 (ClinicalTrials.gov: NCT01600430)[1].
Neurodevelopmental and Respiratory Outcomes at 2 years
This follow-up study was approved by the Institutional Review Board at the University of Alabama at Birmingham. The primary clinical outcome was the cognitive composite score on the Bayley Scales of Infant and Toddler Development, third edition (BSID III). Neurodevelopmental impairment (NDI), NDI or death prior to follow-up, cognitive impairment, language impairment, and use of asthma medications (bronchodilator therapy, inhaled steroids, oral or IV steroids, montelukast, and others) or supplemental oxygen at 22 to 26 months of age were secondary outcomes. Certified examiners who were unaware of group allocation assessed these outcomes.
NDI was defined as any of the following: a cognitive composite score on the BSID III of less than 85, moderate or severe cerebral palsy with a Gross Motor Function Classification System (GMFCS) score of 2 or higher, hearing impairment, or bilateral visual impairment[21]. Cognitive and language impairments were defined, respectively, as a cognitive and language composite score on the BSID III of less than 85.
Although children with missing data on the BSID III were considered lost to follow-up, a surrogate for developmental impairment was used for children with routine follow-up visits at our institution to minimize follow-up bias. Children who were not blind or deaf and were not diagnosed with cerebral palsy or developmental delay on routine pediatric assessments were classified as not having developmental impairment.
Respiratory outcomes at 2 years of age were assessed with a structured questionnaire for parents or primary caregivers that included questions about the use of asthma medications and the use of supplemental oxygen/ventilator, or CPAP (home use or use in a chronic care facility) up to 3 months prior to the follow-up visit.
Statistical analysis
For this follow-up study, a subsample of 80 infants eligible for follow-up was anticipated to yield 80% power for the primary clinical outcome of cognitive scores at 22 to 26 months of age if each comparison group had a minimum of 24 infants and the intervention had an extremely large effect size (10-point difference in cognitive scores). The Wilcoxon test was used to compare medians between supplementation groups. An extended Mantel-Haenszel chi square test for linear trend was used to compare secondary outcomes of this dose-response trial. Vitamin D concentration at birth was analyzed as a covariate. The interaction between supplementation group and vitamin D concentration at birth for the outcome of BSID III cognitive score at 22 to 26 months of age was assessed with interaction terms in a post hoc analysis of covariance using a fixed-effects model. All analyses were performed using the intention-to-treat principle. Imputation techniques for missing outcomes were not used.
RESULTS
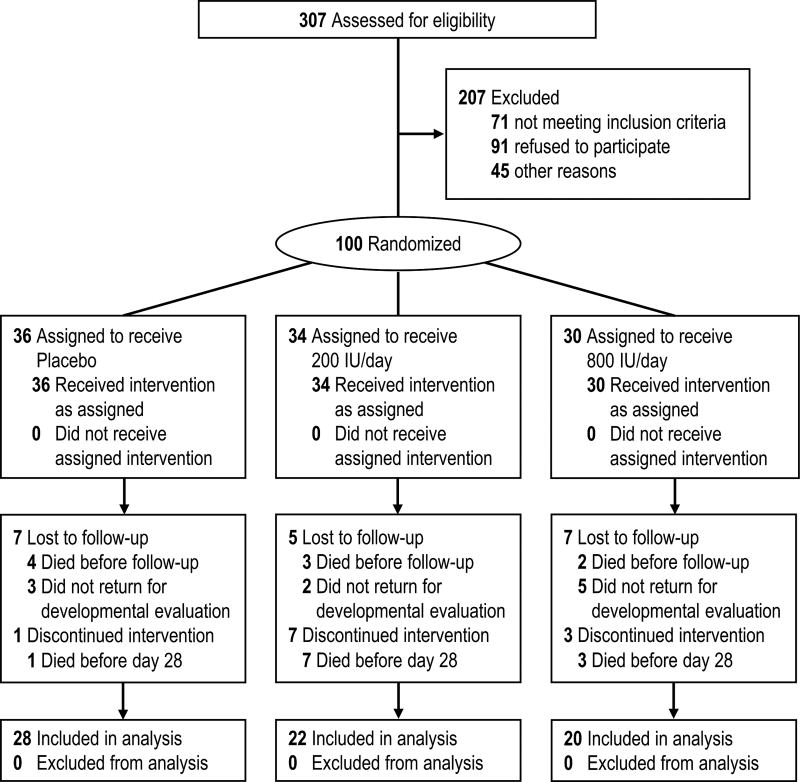
Of the 100 extremely preterm infants enrolled in the trial between 2012 and 2014, 20 died before 22 to 26 months of age (Figure 1).
Figure 1.
Enrollment, randomization, and outcomes
Of the 80 survivors at 22 to 26 months of age, 70 (88%) received comprehensive neurodevelopmental evaluations that included BSID III cognitive scores; 28 of the 31 infants that completed the intervention in the placebo group (90%), 22 of the 24 infants that completed the intervention in the 200 IU group (92%), and 20 of the 25 infants that completed the intervention in the 800 IU group (80%). These follow-up rates did not differ significantly between groups (p=0.39). Additionally, 8 of the 10 children with a missing comprehensive neurodevelopmental evaluation were alive and received regular pediatric assessments after hospital discharge. A surrogate outcome of developmental impairment was thus assessed in these 8 infants. One of these infants was evaluated by Neurology and Rehabilitation Medicine physicians and was diagnosed with moderate cerebral palsy and a GMFCS of 2. The outcome of NDI or death was therefore determined for 91 of the 100 infants enrolled in the trial.
Table 1 summarizes the demographic and clinical characteristics of the supplementation groups at follow-up. Vitamin D concentrations were measured at birth for 56 of the 70 infants assessed with the BSID III at 2 years of age. Vitamin D concentrations were measured around postnatal day 28 for 51 of the 70 infants assessed with the BSID III at 2 years of age.
Table 1.
Demographic and clinical data.
| Placebo Group (n=28) |
200 IU Group (n=22) |
800 IU Group (n=20) |
|
|---|---|---|---|
| GA in weeks, median (IQR) | 26 (24 – 27) | 26 (24 – 27) | 25 (24 –26) |
| BW in grams, mean ± SD | 775 ± 199 | 800 ± 182 | 743 ± 215 |
| Male sex, n/N (%) | 12/28 (43) | 12/22 (55) | 6/20 (30) |
| Black race, n/N (%) | 13/28 (46) | 13/22 (59) | 9/20 (45) |
| Multiple gestation, n/N (%) | 6/28 (9) | 5/22 (7) | 3/20 (4) |
| Public health insurance, n/N (%) | 23/28 (68) | 16/22 (72) | 11/20 (55) |
| Maternal marital status (married), n/N (%) | 10/24 (42) | 8/18 (44) | 11/19 (58) |
| Maternal education (≥ high school), n/N (%) | 23/28 (82) | 18/22 (82) | 18/20 (90) |
| Child living with biological parents, n/N (%) | 14/24 (58) | 7/18 (39) | 10/19 (53) |
| 25(OH)D in nmol/l at birth, median (IQR) | 45 (25 – 60) | 32 (22 – 40) | 40 (25 – 60) |
| 25(OH)D in ng/ml at birth, median (IQR) | 18 (10 – 24) | 13 (9 – 16) | 16 (10 – 24) |
| Vitamin D deficiency at birth defined as 25(OH)D < 20 ng/ml | 15/23 (65) | 15/17 (88) | 10/16 (62) |
| Vitamin D deficiency at birth defined as 25(OH)D < 12 ng/ml | 8/23 (35) | 5/17 (29) | 6/16 (38) |
| Postnatal day of first enteral feeding (including buccal colostrum), median (IQR) | 2 (1 – 3) | 2 (1 – 3) | 2 (1 – 3) |
| Postnatal day of first enteral dose, median (IQR) | 5 (5 – 6) | 5 (5 – 6) | 5 (5 – 6) |
| Head circumference in cm at 36 weeks, median (IQR) | 30 (28 – 31) | 30 (29 – 32) | 30 (29 – 31) |
| 25(OH)D in ng/ml at 28 days, median (IQR)* | 24 (14 – 48) | 30 (18 – 42) | 83 (50 – 99) |
| 25(OH)D in nmol/l at 28 days, median (IQR)* | 60 (35 – 119) | 75 (45 – 105) | 208 (125 – 247) |
n=51: 21 in the placebo group, 17 in the 200 IU group, and 13 in the 800 IU group.
The median BSID III cognitive score was 90 (IQR: 80 – 100). There were no significant differences in the primary outcome between supplementation groups (Table 2). The median BSID III cognitive score was not significantly higher in the 800 IU group than in the placebo group (median difference favoring the 800 IU group: +5 points; 95% CI −5 to 15; p=0.23). The post hoc analysis with adjustment for vitamin D concentrations at birth using a fixed-effect model did not show significant differences in adjusted BSID III cognitive scores across supplementation groups (p=0.11). The adjusted mean BSID III cognitive score was 94 in the 800 IU group and 86 in the placebo group (adjusted mean difference favoring the 800 IU group: +8 points; 95% CI −1 to 19; p=0.06). An additional post-hoc analysis of the association between early vitamin D supplementation and cognitive scores at 2 years of age adjusted for sociodemographic factors, bronchopulmonary dysplasia (BPD), intraventricular hemorrhage, and late-onset sepsis was not significantly different than the unadjusted analysis or the analysis with adjustment for vitamin D concentrations at birth. BPD at 36 weeks was the only covariate that showed a significant association with lower cognitive scores at 2 years of age.
Table 2.
Neurodevelopmental and respiratory outcomes at 2 years of age expressed as unadjusted proportions, risk ratios, and median differences.
| Placebo Group |
200 IU Group |
800 IU Group |
p | 800 IU/day vs. placebo |
200 IU/day vs. placebo |
|
|---|---|---|---|---|---|---|
| Cognitive score, median (IQR) | 90 (80 – 99) | 90 (70 – 100) | 95 (80 – 100) | 0.47 | MD‡: 5 (95% CI −5 to 15;33333 p=0.23) | MD: 0 (95% CI −10 to 10; p=0.98) |
| NDI, n/N (%) | 15/28 (54) | 10/23 (43) | 6/20 (30) | 0.08 | RR: 0.6 (95% CI: 0.3 to 1.2; p=0.10) | RR: 0.8 (95% CI: 0.5 to 1.4; p=0.47) |
| NDI or surrogate of developmental impairment†, n/N (%) | 15/30 (50) | 11/24 (46) | 7/24 (29) | 0.10 | RR: 0.6 (95% CI: 0.3 to 1.2; p=0.12) | RR: 0.9 (95% CI: 0.5 to 1.6; p=0.76) |
| NDI or death, n/N (%) | 20/33 (61) | 20/33 (61) | 11/25 (44) | 0.18 | RR: 0.7 (95% CI: 0.4 to 1.2; p=0.21) | RR: 1.0 (95% CI: 0.7 to 1.5; p=0.99) |
| Cognitive impairment, n/N (%) | 11/28 (39) | 8/22 (36) | 6/20 (30) | 0.42 | RR: 0.8 (95% CI: 0.3 to 1.7; p=0.50) | RR: 0.9 (95% CI: 0.5 to 1.9; p=0.83) |
| Language impairment, n/N (%) | 18/28 (64) | 12/21 (57) | 9/20 (45) | 0.15 | RR: 0.7 (95% CI: 0.4 to 1.2; p=0.13) | RR: 0.9 (95% CI: 0.6 to 1.4; p=0.61) |
| Language score, median (IQR) | 79 (74 – 91) | 79 (64 – 91) | 86 (78 – 93) | 0.19 | MD: 5 (95% CI: −3 to 12; p=0.22) | MD: 4 (95% CI: −14 to 6; p=0.35) |
| Use of asthma medications up to 3 months prior to follow-up visit, n/N (%) | 13/29 (44) | 10/23 (43) | 9/22 (41) | 0.67 | RR: 0.9 (95% CI: 0.5 to 1.7; p=0.78) | RR: 1.0 (95% CI: 0.5 to 1.8; p=0.92) |
| Use of supplemental oxygen up to 3 months prior to follow-up visit, n/N (%) | 2/28 (7) | 2/23 (9) | 0/21 (0) | 0.19 | RR: incalculable | RR: 1.2 (95% CI: 0.2 to 8.0; p=0.84) |
| Mortality, n/N (%) | 5/36 (14) | 10/34 (29) | 5/30 (17) | 0.23 | RR: 2.1 (95% CI: 0.8 to 5.6; p=0.12) | RR: 1.2 (95% CI: 0.4 to 3.8; p=0.76) |
MD: Median difference. RR: Risk ratio. CI: Confidence intervals. IQR: Interquartile range
To minimize the risk of follow-up bias, the outcomes of children who did not complete BSID III evaluations but had follow-up data are reported (n=78). If the child was not blind or deaf and was not diagnosed with cerebral palsy or developmental delay on routine pediatric assessments, the child was classified as not having developmental impairment.
Median difference was reported because normal distribution of cognitive scores in subgroups of less than 30 infants could not be assumed.
The dose-response effect of early vitamin D supplementation on reduction of NDI was not statistically significant (p=0.08). Compared with placebo, higher vitamin D doses did not significantly reduce NDI (p=0.10) or other surrogates of developmental impairment (p=0.12). Similarly, the outcome of NDI or death at 22 to 26 months of age did not significantly differ between supplementation groups (p=0.18).
Cognitive impairment and language impairment among surviving infants did not differ significantly between supplementation groups. The median BSID III language score was 79 (IQR: 73 – 91). The BSID III language score was not significantly higher in the 800 IU group (median difference favoring the 800 IU group: +5 points; 95% CI −3 to 12; p=0.22).
The use of asthma medications or supplemental oxygen up to 3 months prior to follow-up at 22 to 26 months of age did not significantly decrease with early vitamin D supplementation.
DISCUSSION
This is a 2-year follow-up study to assess neurodevelopmental and respiratory outcomes of extremely preterm infants supplemented with vitamin D in the first 28 days after birth. In this phase II single-center trial, infants randomly assigned to receive higher doses of vitamin D supplementation did not have significantly higher cognitive scores at 22 to 26 months of age than infants receiving lower vitamin D doses or placebo. Respiratory outcomes did not differ between groups. Early vitamin D supplementation may have a dose-response effect on reduction in NDI across supplementation groups, but this linear trend did not reach statistical significance [22]. The analysis with interaction terms between vitamin D status at birth and cognitive scores at 22 to 26 months of age revealed that extremely preterm infants with lower vitamin D concentrations at birth may benefit from supplementation with high doses of vitamin D, though there are concerns for vitamin D concentrations outside the suggested therapeutic range.
The linear trend between higher vitamin D doses and lower NDI was not statistically significant most likely because the sample size was small and vitamin D concentrations at postnatal day 28 in the 200 IU and placebo groups were equally low. While some trials have concluded that lower vitamin D doses are sufficient to achieve vitamin D concentrations within normal range[23], this trial and others have shown that enteral supplementation with higher doses of vitamin D is well tolerated in infants and results in higher vitamin D concentrations after a few weeks of supplementation[1,24,25].
Neither interventional[12,19,20] nor observational studies[2,3] have reported on early childhood outcomes of preterm infants after vitamin D supplementation. Most clinical studies have related neurocognitive outcomes of children to vitamin D status either during pregnancy[17,26–28] or at the time of birth[15,29,30]. Higher maternal vitamin D concentrations early in gestation (between 14 and 18 weeks of gestation) have been a ssociated with higher mental and psychomotor scores[26] and lower frequency of language impairment[17] in infants delivered at term and assessed between 14 and 24 months of age. In contrast, higher maternal vitamin D concentrations late in gestation (between 30 and 32 weeks of gestation) have not been associated with improved cognitive function or school performance in infants delivered at term and assessed during childhood[27,28]. The association between vitamin D concentration in cord blood and neurocognitive outcomes of infants delivered at term is more heterogeneous. While some authors report that low and high vitamin D concentrations in cord blood are associated with mental delay in infants delivered at term[15], others have observed very little association between vitamin D concentrations in cord blood and cognitive development in term infants[30]. A recent observational study relating vitamin D concentrations in cord blood to neurocognitive outcomes of children showed that vitamin D concentrations in the range of sufficiency were marginally associated with higher BSID III cognitive and language scores after adjustment for potential confounders[29].
One of the most important limitations of this follow-up study is that early childhood outcomes were not prespecified at the time of trial registration and, therefore, the study was not adequately powered to assess these outcomes. These important clinical outcomes were added later to attenuate the increasing problem of disproportion between observational studies suggesting benefits of vitamin D on neurodevelopment and randomized trials reporting these effects. The expected tradeoffs were limited power for these outcomes and reduced precision, strength, and statistical significance of our risk estimates.
The number of infants who died (20) and those lost to follow-up (10) were another limitation. This limitation affected the final number of infants available for analysis of neurodevelopmental and respiratory outcomes. Other limitations in study design include duration of supplementation, lack of data on other biomarkers of vitamin D metabolism, lack of data on vitamin D concentrations at 2 years of age, assumption of equivalence between moderate and severe NDI, and use of unadjusted median values.
Strengths of the study include dose-response design that included a placebo plus two levels of supplementation for inferences of causality based on linear trends, masking procedures, use of the intention-to-treat principle to preserve the effect of randomization, use of broad inclusion criteria to increase generalizability, comprehensive follow-up of infants enrolled in a neonatal trial, and report of effect estimates and confidence intervals to allow clinicians to infer the scientific value of the results themselves[22]. This phase II trial of early vitamin D supplementation also provides critical information for designing a larger phase III multicenter clinical trial. Our results not only reveal that early vitamin D supplementation with 200 IU/day is insufficient to increase vitamin D concentrations to a range associated with vitamin D sufficiency but also suggest that early vitamin D supplementation with 800 IU/day might be necessary to rapidly correct low vitamin D concentrations at birth and improve cognitive scores in extremely preterm infants. Since supplementation with 800 IU/day for 4 weeks resulted in vitamin D concentrations that greatly exceeded recommended targets, more information is needed to assess the appropriateness of this high dose over time. An alternative method of supplementation in which the initial dose of 800 IU/day is gradually reduced based on weekly vitamin D measurements may be needed to facilitate rapid correction without increasing the risk of vitamin D toxicity. Early vitamin D supplementation is a reasonably safe and low-cost intervention that may attenuate NDI. A larger parallel-group randomized trial powered to detect a more realistic 5-point difference on cognitive scores between groups would require approximately 300 extremely preterm infants if indirect effects of early vitamin D supplementation on reduction of BPD are not anticipated.
Conclusions
In summary, early vitamin D supplementation did not significantly improve BSID III cognitive scores in extremely preterm infants. The quantitative effects of vitamin D on NDI, language impairment, and need for supplemental oxygen at 2 years of age suggest a dose-response relationship, but this phase II trial was underpowered to detect small differences between groups that could be considered clinically meaningful.
Acknowledgments
Funding Source: This trial was partially supported by the Kaul Pediatric Research Institute Senior Investigator Award. Drs. Ambalavanan and Carlo are also supported by the National Institutes of Health (NIH), grant number NIH U10 HD34216.
Abbreviations
- NDI
neurodevelopmental impairment
- BSID III
Bayley Scales of Infant and Toddler Development, third edition
- GMFCS
Gross Motor Function Classification System
Footnotes
Conflict of interest: The authors have indicated they have no potential conflicts of interest to disclose.
Clinical Trial Registration: ClinicalTrials.gov: NCT01600430
- AS conceptualized and designed the initial trial and this ancillary study, collected the data, performed statistical analysis of the data, drafted the initial manuscript, revised the manuscript and approved the final manuscript as submitted.
- TW and VP assisted with the study design of this ancillary study, collected the data, revised the manuscript and approved the final manuscript as submitted.
- MP, WC and NA assisted with the study design, critically reviewed the manuscript and approved the final manuscript as submitted.
References
- 1.Fort P, Salas AA, Nicola T, Craig CM, Carlo WA, Ambalavanan N. A Comparison of 3 Vitamin D Dosing Regimens in Extremely Preterm Infants: A Randomized Controlled Trial. J Pediatr. 2016;174:132–138 e131. doi: 10.1016/j.jpeds.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCarthy RA, McKenna MJ, Oyefeso O, Uduma O, Murray BF, Brady JJ, Kilbane MT, Murphy JF, Twomey A, CP OD, Murphy NP, Molloy EJ. Vitamin D nutritional status in preterm infants and response to supplementation. Br J Nutr. 2013;110:156–163. doi: 10.1017/S0007114512004722. [DOI] [PubMed] [Google Scholar]
- 3.Burris HH, Van Marter LJ, McElrath TF, Tabatabai P, Litonjua AA, Weiss ST, Christou H. Vitamin D status among preterm and full-term infants at birth. Pediatr Res. 2014;75:75–80. doi: 10.1038/pr.2013.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner CL, Greer FR American Academy of Pediatrics Section on B; American Academy of Pediatrics Committee on N. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142–1152. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 5.Taylor SN, Hollis BW, Wagner CL. Vitamin D needs of preterm infants. NeoReviews. 2009;10:e590–599. [Google Scholar]
- 6.Thandrayen K, Pettifor JM. Maternal vitamin D status: implications for the development of infantile nutritional rickets. Endocrinol Metab Clin North Am. 2010;39:303–320. doi: 10.1016/j.ecl.2010.02.006. table of contents. [DOI] [PubMed] [Google Scholar]
- 7.Agostoni C, Buonocore G, Carnielli VP, De Curtis M, Darmaun D, Decsi T, Domellof M, Embleton ND, Fusch C, Genzel-Boroviczeny O, Goulet O, Kalhan SC, Kolacek S, Koletzko B, Lapillonne A, Mihatsch W, Moreno L, Neu J, Poindexter B, Puntis J, Putet G, Rigo J, Riskin A, Salle B, Sauer P, Shamir R, Szajewska H, Thureen P, Turck D, van Goudoever JB, Ziegler EE. Nutrition ECo: Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2010;50:85–91. doi: 10.1097/MPG.0b013e3181adaee0. [DOI] [PubMed] [Google Scholar]
- 8.Kovacs CS. Vitamin D in pregnancy and lactation: maternal, fetal, and neonatal outcomes from human and animal studies. Am J Clin Nutr. 2008;88:520S–528S. doi: 10.1093/ajcn/88.2.520S. [DOI] [PubMed] [Google Scholar]
- 9.Abrams SA. Committee on N: Calcium and vitamin d requirements of enterally fed preterm infants. Pediatrics. 2013;131:e1676–1683. doi: 10.1542/peds.2013-0420. [DOI] [PubMed] [Google Scholar]
- 10.Hollis BW, Wagner CL, Howard CR, Ebeling M, Shary JR, Smith PG, Taylor SN, Morella K, Lawrence RA, Hulsey TC. Maternal Versus Infant Vitamin D Supplementation During Lactation: A Randomized Controlled Trial. Pediatrics. 2015;136:625–634. doi: 10.1542/peds.2015-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 12.Backstrom MC, Maki R, Kuusela AL, Sievanen H, Koivisto AM, Ikonen RS, Kouri T, Maki M. Randomised controlled trial of vitamin D supplementation on bone density and biochemical indices in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1999;80:F161–166. doi: 10.1136/fn.80.3.f161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zosky GR, Hart PH, Whitehouse AJ, Kusel MM, Ang W, Foong RE, Chen L, Holt PG, Sly PD, Hall GL. Vitamin D deficiency at 16 to 20 weeks' gestation is associated with impaired lung function and asthma at 6 years of age. Ann Am Thorac Soc. 2014;11:571–577. doi: 10.1513/AnnalsATS.201312-423OC. [DOI] [PubMed] [Google Scholar]
- 14.Walker VP, Modlin RL. The vitamin D connection to pediatric infections and immune function. Pediatr Res. 2009;65:106R–113R. doi: 10.1203/PDR.0b013e31819dba91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu P, Tong SL, Hao JH, Tao RX, Huang K, Hu WB, Zhou QF, Jiang XM, Tao FB. Cord blood vitamin D and neurocognitive development are nonlinearly related in toddlers. J Nutr. 2015;145:1232–1238. doi: 10.3945/jn.114.208801. [DOI] [PubMed] [Google Scholar]
- 16.Eyles D, Brown J, Mackay-Sim A, McGrath J, Feron F. Vitamin D3 and brain development. Neuroscience. 2003;118:641–653. doi: 10.1016/s0306-4522(03)00040-x. [DOI] [PubMed] [Google Scholar]
- 17.Whitehouse AJ, Holt BJ, Serralha M, Holt PG, Kusel MM, Hart PH. Maternal serum vitamin D levels during pregnancy and offspring neurocognitive development. Pediatrics. 2012;129:485–493. doi: 10.1542/peds.2011-2644. [DOI] [PubMed] [Google Scholar]
- 18.Pet MA, Brouwer-Brolsma EM. The Impact of Maternal Vitamin D Status on Offspring Brain Development and Function: a Systematic Review. Adv Nutr. 2016;7:665–678. doi: 10.3945/an.115.010330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Natarajan CK, Sankar MJ, Agarwal R, Pratap OT, Jain V, Gupta N, Gupta AK, Deorari AK, Paul VK, Sreenivas V. Trial of daily vitamin D supplementation in preterm infants. Pediatrics. 2014;133:e628–634. doi: 10.1542/peds.2012-3395. [DOI] [PubMed] [Google Scholar]
- 20.Hanson C, Jones G, Lyden E, Kaufmann M, Armas L, Anderson-Berry A. Vitamin D metabolism in the premature newborn: A randomized trial. Clin Nutr. 2016;35:835–841. doi: 10.1016/j.clnu.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 21.Vaucher YE, Peralta-Carcelen M, Finer NN, Carlo WA, Gantz MG, Walsh MC, Laptook AR, Yoder BA, Faix RG, Das A, Schibler K, Rich W, Newman NS, Vohr BR, Yolton K, Heyne RJ, Wilson-Costello DE, Evans PW, Goldstein RF, Acarregui MJ, Adams-Chapman I, Pappas A, Hintz SR, Poindexter B, Dusick AM, McGowan EC, Ehrenkranz RA, Bodnar A, Bauer CR, Fuller J, O'Shea TM, Myers GJ, Higgins RD. Network SSGotEKSNNR: Neurodevelopmental outcomes in the early CPAP and pulse oximetry trial. N Engl J Med. 2012;367:2495–2504. doi: 10.1056/NEJMoa1208506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kyriacou DN. The Enduring Evolution of the P Value. JAMA. 2016;315:1113–1115. doi: 10.1001/jama.2016.2152. [DOI] [PubMed] [Google Scholar]
- 23.Siafarikas A, Piazena H, Feister U, Bulsara MK, Meffert H, Hesse V. Randomised controlled trial analysing supplementation with 250 versus 500 units of vitamin D3, sun exposure and surrounding factors in breastfed infants. Arch Dis Child. 2011;96:91–95. doi: 10.1136/adc.2009.178301. [DOI] [PubMed] [Google Scholar]
- 24.Gallo S, Comeau K, Vanstone C, Agellon S, Sharma A, Jones G, L'Abbe M, Khamessan A, Rodd C, Weiler H. Effect of different dosages of oral vitamin D supplementation on vitamin D status in healthy, breastfed infants: a randomized trial. JAMA. 2013;309:1785–1792. doi: 10.1001/jama.2013.3404. [DOI] [PubMed] [Google Scholar]
- 25.Holmlund-Suila E, Viljakainen H, Hytinantti T, Lamberg-Allardt C, Andersson S, Makitie O. High-dose vitamin d intervention in infants--effects on vitamin d status, calcium homeostasis, and bone strength. J Clin Endocrinol Metab. 2012;97:4139–4147. doi: 10.1210/jc.2012-1575. [DOI] [PubMed] [Google Scholar]
- 26.Morales E, Guxens M, Llop S, Rodriguez-Bernal CL, Tardon A, Riano I, Ibarluzea J, Lertxundi N, Espada M, Rodriguez A, Sunyer J. Project I: Circulating 25-hydroxyvitamin D3 in pregnancy and infant neuropsychological development. Pediatrics. 2012;130:e913–920. doi: 10.1542/peds.2011-3289. [DOI] [PubMed] [Google Scholar]
- 27.Strom M, Halldorsson TI, Hansen S, Granstrom C, Maslova E, Petersen SB, Cohen AS, Olsen SF. Vitamin D measured in maternal serum and offspring neurodevelopmental outcomes: a prospective study with long-term follow-up. Ann Nutr Metab. 2014;64:254–261. doi: 10.1159/000365030. [DOI] [PubMed] [Google Scholar]
- 28.Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, Godfrey KM, Cooper C. Princess Anne Hospital Study G: Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. 2008;62:68–77. doi: 10.1038/sj.ejcn.1602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gould JF, Anderson AJ, Yelland LN, Smithers LG, Skeaff CM, Zhou SJ, Gibson RA, Makrides M. Association of cord blood vitamin D with early childhood growth and neurodevelopment. J Paediatr Child Health. 2016 doi: 10.1111/jpc.13308. [DOI] [PubMed] [Google Scholar]
- 30.Keim SA, Bodnar LM, Klebanoff MA. Maternal and cord blood 25(OH)-vitamin D concentrations in relation to child development and behaviour. Paediatr Perinat Epidemiol. 2014;28:434–444. doi: 10.1111/ppe.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]