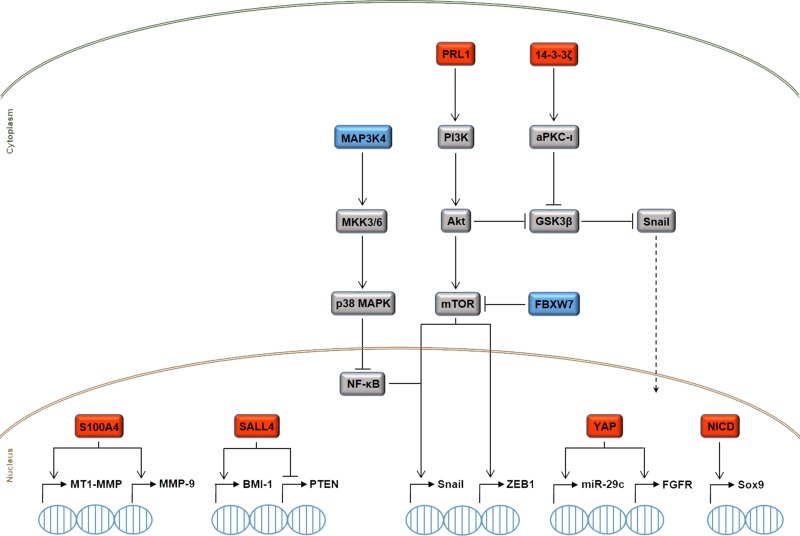
Figure 2.
Simplified overview of the intracellular signaling mechanisms governing CCA cell motility and invasiveness. The upregulation (red boxes) or downregulation (blue boxes) of different transcription factors [S100A4, Sal-like protein 4 (SALL4), Yes-associated protein (YAP), Notch intracellular domain (NICD)], protein kinases [mitogen-activated protein kinase kinase kinase 4 (MAP3K4)] and phosphatases [phosphatases of regenerating liver 1 (PRL1)], ubiquitin ligases [F-box and WD repeat domain-containing 7 (FBXW7)], and adaptor proteins (14-3-3ζ) is responsible for the emergence of proinvasive features in CCA cells, due to the activation of deleterious transcriptional programs that are closely interwoven with the epithelial-to-mesenchymal transition (EMT) process. Protein tyrosine phosphatases protein tyrosine phosphatase nonreceptor 3 (PTPN3) and eyes absent homolog 4 (EYA4) are also endowed with protumorigenic and antitumorigenic functions in CCA, respectively, but the underlying molecular mechanisms have yet to be unveiled. Because of space limitations, we could not fully illustrate the extensive crosstalk among the depicted pathways. For instance, miR-29c, which is upregulated by YAP, can negatively regulate the expression of phosphatase and tensin homolog (PTEN), thereby allowing Akt to upregulate the expression of the oncoprotein gankyrin. Moreover, BMI-1 upregulation by SALL4 supports the activation of the prometastatic Wnt pathway. See text for further details. aPKC)-ι, atypical protein kinase C-ι; FGFR, fibroblast growth factor receptor; GSK-3β, lycogen synthase kinase-3β; mTOR, mammalian target of rapamycin; MMP-9, matrix metalloproteinase-9; MT1-MMP, membrane-type 1-MMP; NF-κB, nuclear factor-κB; Sox9, sex-determining region Y-box 9.