Figure 1. The influence of LL-37 citrullination on DNA binding.
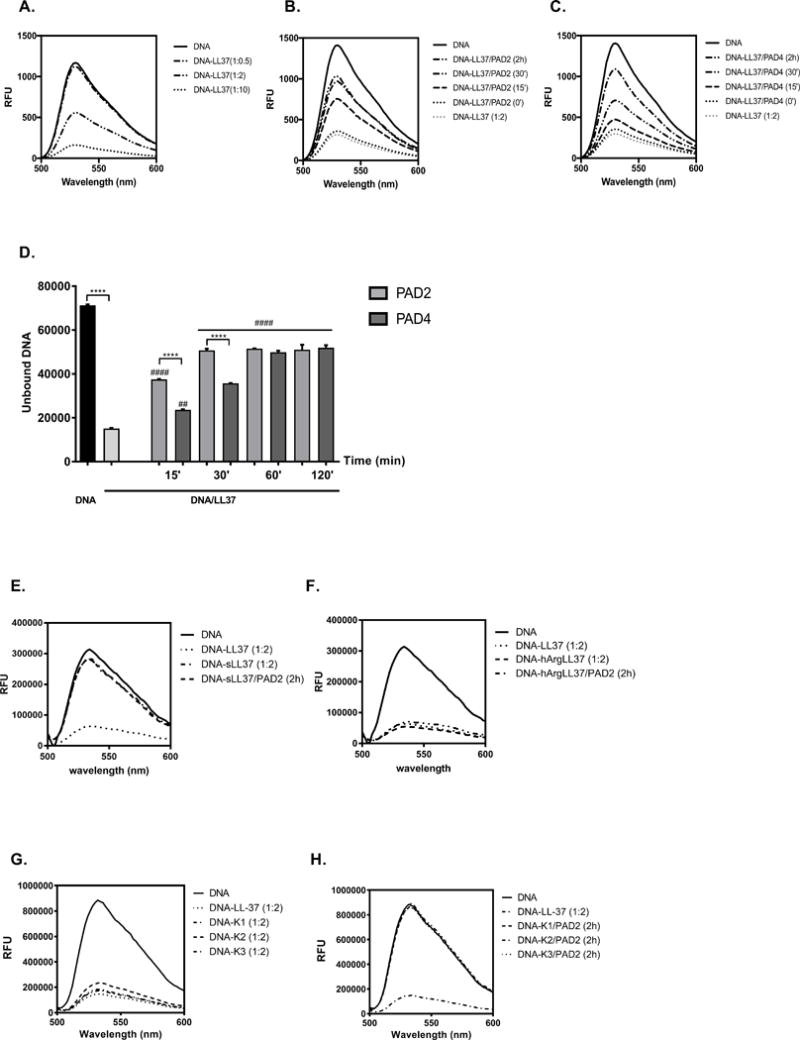
(A) The optimal binding between DNA and LL-37 was established by incubation of molecules at the different molar ratio of 1:0.5, 1:2, 1:10 (DNA:peptide) for 10 min at room temperature. Unbound DNA was quantified with spectral analysis using PicoGreen and is shown as relative fluorescence units (RFU). (B, C, D) The time dependent effect of LL-37 incubation with PAD2 (B) and PAD4 (C) on peptide capability to bind DNA. Statistical significance was evaluated by one-way ANOVA, followed by Tukey’s multiple comparisons post-test (****p<0.000 – comparing to control; ##p<0.01; ####p<0.0001- comparing with DNA/LL-37). (E) The DNA binding to the untreated scrambled peptide (sLL37) and after its citrullination with PAD2. (F) The DNA binding by LL-37 with Arg residues substituted with homoarginine (hArg-LL-37) with or without pretreatment with PAD2. (G) The binding of carbamylated variants of LL-37 to DNA before and after (H) preincubation with PAD2.
