Abstract
Objectives
To characterize uptake and correlates of effective contraceptive use postpartum.
Study Design
We analyzed data from a national, cross-sectional evaluation of prevention of mother-to-child HIV transmission programs that enrolled women attending 6-week or 9-month infant immunization visits at 120 Kenyan maternal and child health clinics. We classified women who resumed sexual activity postpartum and did not desire a child within 2 years as having a need for family planning (FP).
Results
We included 955 (94%) of 1012 women 8–10 months postpartum in the analysis. Mean age was 25.8 years and 36% were primigravidas. By 9-months postpartum 62% of all women used contraception and 59% used effective contraception (injectables, implants, intrauterine devices [IUDs], oral contraceptives [OCs], and tubal ligations). Most contraceptive users (61%) used injectables, followed by implants (10%), OCs (6%), IUDs (4%), and condoms alone (2%). The majority (n=733, 77%) had a need for FP and 67% of 733 women with FP need used effective contraception. Among women with a need for FP, effective contraception use was higher among those who discussed FP in postnatal care (PNC) than who did not discuss FP in PNC (Prevalence Ratio (PR) for PNC alone: 1.35 (95% Confidence Interval [CI]:1.16-1.58; PR for PNC and antenatal care [ANC]:1.42, 95% CI: 1.21-1.67; p=0.001 for both).
Conclusions
Two-thirds of postpartum women with a need for FP used effective contraception at 9-months postpartum, and use was associated with discussing FP during PNC.
Implications
Integrating FP counseling in ANC/PNC could be an effective strategy to increase effective contraception use.
Keywords: postpartum, contraception, Kenya, maternal health, child health
1.0 INTRODUCTION
Reducing unmet need for family planning (FP) is a global public health priority, and a critical component of the post-2015 sustainable development goals (SDGs) and FP2020 initiative.[1, 2] Postpartum women benefit from FP as a strategy to plan and space their births. Short interpregnancy intervals are associated with higher risk of infant mortality, preterm birth, low birthweight, and small-for-gestational-age.[3, 4] Using effective FP methods helps extend the interpregnancy interval to reduce these maternal and child health (MCH) risks. Yet, unmet need for FP during the postpartum period remains high. Data from women ≤2 years postpartum participating in Demographic and Health Surveys (DHS) in low- and bottom- income countries (LMIC) suggest unmet need exceeds 60% using a prospective definition of unmet need.[5] In Kenya, unmet need has declined among the general population between 2008 and 2014; however, more recent data on unmet need during the postpartum period are lacking.[6] In addition, method mix in Kenya has shifted during this same time period with higher use of implants, but temporal changes and correlates of specific FP methods have not been well characterized among postpartum Kenyan women.
Since over one-third of women in LMICs introduce complementary infant feeds before 6 months postpartum, effectiveness of the lactational amenorrhea method (LAM) for pregnancy prevention is diminished.[7] Thus, it is essential for postpartum women to receive FP services and effective methods early to achieve healthy birth spacing and prevent unintended pregnancies. Previous studies report that women who attend more antenatal care (ANC) visits, deliver with a skilled birth attendant, or attend postnatal care (PNC) are more likely to use postpartum contraception.[8–12] However, other factors may also impact use of FP during the postpartum period.
We characterized uptake and correlates of effective contraception, and specific types of FP, using data from a survey of postpartum women sampled from 120 facilities across Kenya.
2.0 MATERIAL AND METHODS
2.1 Study design and sampling
In 2013, we conducted a cross-sectional study in MCH clinics providing ANC and PNC throughout Kenya to assess coverage and uptake of prevention of mother-to-child HIV transmission (PMTCT) services as previously described.[13] Briefly, we used probability proportionate to size sampling to select 121 MCH clinics in seven of the eight geographical regions in Kenya from which all mother-infant pairs were sampled to participate during a 5-day period per clinic. We classified facility size based on annual number of ANC clinic visits in 2011 and excluded “small” (<500 visits) and/or North Eastern Province facilities due to logistical constraints. (Figure 1).
Figure 1. Study locations (n=120) and regional uptake of effective contraceptive methods among Kenyan postpartum women attending maternal and child health clinics, 2013.
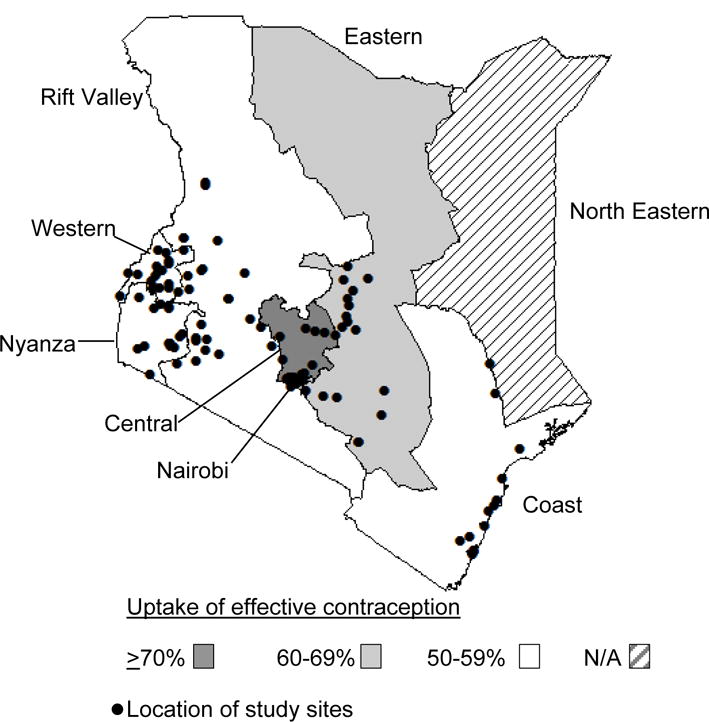
Percentages are weighted
2.2 Study procedures
Study nurses enrolled women attending routine 6-week or 9-month immunization visits and administered a survey on demographic and clinical characteristics, reproductive and medical history, and partner characteristics. We asked women whether they were trying to become pregnant or prevent pregnancy when they became pregnant, about their future fertility intentions, and about their current FP use. Women were also asked about whether anyone has talked to them about FP, and if yes, the timing and setting of the discussion (ANC, PNC, non-ANC/PNC, non-health care setting, family/community, or other setting). The Hurt/Insult/Threaten/Scream (HITS) scale was used to determine history of intimate partner violence (IPV).[14]
Ethical review committees at the Kenya Medical Research Institute and the University of Washington, and the Associate Director for Science at the U.S. Centers for Disease Control and Prevention, approved all study procedures.
2.3 Contraceptive definitions and statistical analysis
Modern contraception included condoms, injectables, implants, intrauterine devices (IUDs), oral contraceptives (OCs), and tubal ligations (TL). Long-acting, reversible contraception (LARC) included IUDs and implants. Effective contraception included all modern contraceptives except condoms alone. [15–17]. Dual method use consisted of condoms plus another effective contraceptive method. We defined women with a need for FP as those who resumed sexual activity after delivery and did not desire a child within the next two years; women with an unmet need for FP were women with a need for FP who were not using modern contraception. We identified correlates of effective contraceptive use among women who had a need for FP using Chi-squared tests for proportions and T-tests for continuous measures and univariate Poisson generalized linear models with a log-link function, an approach used when the outcome prevalence is high.[18, 19] We applied survey weights and clinic-level clustering adjustments to account for the sampling design and ensure representativeness; reported percentages are weighted. Variables significant at p<0.10 were included in a multivariate Poisson generalized linear model. We included the variable with the least amount of data missing in multivariate models if variables were collinear. Variables with p>0.05 were removed from the final multivariate model. We used Stata 13.1/MP svy commands (Stata Corporation, College Station, TX) to perform statistical analyses.
3.0 RESULTS
In total, 955 (94%) of 1012 women 8–10 months postpartum attending 9-month infant immunization visits were included in the analysis (Figure 2). Mean age was 25.8 years (standard deviation [SD] 5.8) and mean years of completed education was 9.0 (SD 3.7) (Table 1). Most (88%) women were married, with mean relationship duration of 5.7 years (SD 4.9) among women with current partners. Overall, HIV prevalence was 7%. Over one-third (36%) of women were primigravidas and the mean number of living children was 2.4 (SD 1.7). Only half of women had attended ≥4 ANC clinic visits and 69% delivered in a facility (Table 1).
Figure 2. Study flowchart for Kenyan postpartum women attending maternal and child health clinics, 2013.
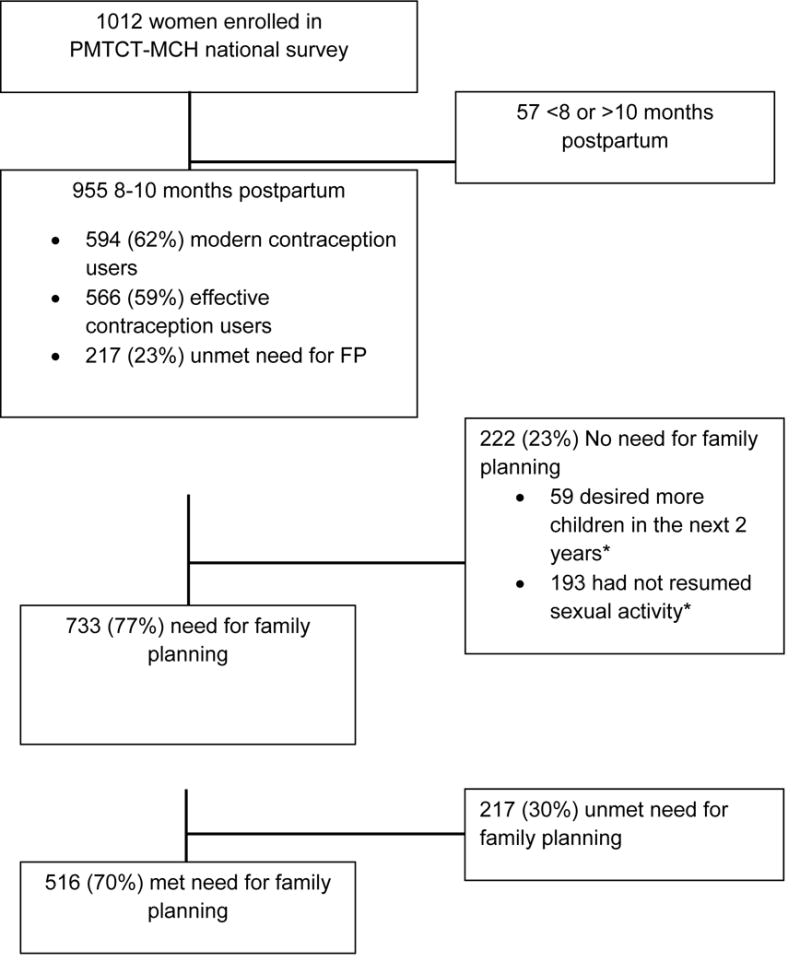
*Not mutually exclusive. PMTCT=prevention of mother-to-child HIV transmission; MCH=maternal and child health Modern contraception included condoms, injectables, implants, intrauterine devices, oral contraceptives, and tubal ligations; effective contraception included all modern contraception except condoms. Women with unmet need reported resuming sexual activity after delivery and not desiring children within the next two years. Percentages are weighted.
Table 1.
Characteristics of Kenyan postpartum women attending maternal and child health clinics participating in the study, by effective contraceptive use, 20131
| N2 | Weight Mean (SD) or Unweighted N (Weighted %)
|
||||||
|---|---|---|---|---|---|---|---|
| All women (n=955) |
Effective contraceptive use
|
||||||
| No (n=389) |
Yes (n=566) |
||||||
|
|
|||||||
| Sociodemographic | |||||||
|
| |||||||
| Age (years) | 955 | 26.1 | (5.7) | 26.5 | (6.2) | 25.8 | (5.2) |
| Age category (years) | |||||||
| <19 | 955 | 83 | (8%) | 39 | (9%) | 44 | (8%) |
| 20-34 | 955 | 776 | (82%) | 296 | (77%) | 480 | (85%) |
| ≥35 | 955 | 96 | (10%) | 54 | (14%) | 42 | (7%) |
| Completed education (years) | 955 | 9.5 | (3.8) | 8.7 | (4.3) | 10.0 | (3.4) |
| Both parents alive (vs. one or both deceased) | 951 | 577 | (61%) | 213 | (55%) | 364 | (65%) |
| Married | 955 | 827 | (87%) | 305 | (79%) | 522 | (92%) |
| Monthly household income ≥10,000 KSH | 606 | 174 | (29%) | 52 | (24%) | 122 | (33%) |
|
| |||||||
| Relationships and sexual behavior | |||||||
|
| |||||||
| Relationship duration (years)3 | 843 | 5.5 | (4.6) | 6.0 | (5.2) | 5.2 | (4.2) |
| Partner age difference (years older)3 | 747 | 5.6 | (5.0) | 5.9 | (5.5) | 5.4 | (4.7) |
| ≥1 current sexual partners | 843 | 36 | (4%) | 8 | (2%) | 28 | (5%) |
| Number of lifetime sexual partners | 876 | 1.9 | (1.2) | 1.8 | (1.0) | 1.9 | (1.2) |
| Intimate partner violence (within the past year) | 918 | 379 | (41%) | 144 | (39%) | 235 | (42%) |
| Partner HIV status known 3 | 843 | 570 | (67%) | 191 | (61%) | 379 | (71%) |
| Partner HIV status3 | |||||||
| Negative | 843 | 535 | (63%) | 176 | (57%) | 359 | (68%) |
| Positive | 843 | 35 | (4%) | 15 | (4%) | 20 | (4%) |
| Unknown | 843 | 272 | (33%) | 122 | (39%) | 151 | (29%) |
| Resumed sex since delivery | 940 | 772 | (82%) | 263 | (69%) | 509 | (91%) |
|
| |||||||
| Reproductive history | |||||||
|
| |||||||
| Gravidity | 955 | 2.4 | (1.7) | 2.7 | (2.1) | 2.2 | (1.4) |
| Number of living children | 955 | 2.2 | (1.5) | 2.5 | (1.8) | 2.0 | (1.3) |
| Number of ANC visits | 885 | 3.6 | (1.6) | 3.4 | (1.5) | 3.7 | (1.6) |
| Partner attended ANC | 906 | 322 | (28%) | 98 | (41%) | 224 | (36%) |
| Facility delivery | 955 | 711 | (74%) | 256 | (66%) | 455 | (80%) |
| No desire for more children within 2 years | 955 | 906 | (95%) | 365 | (94%) | 541 | (96%) |
| Tried to become pregnant (last pregnancy) | 952 | 593 | (63%) | 225 | (59%) | 368 | (65%) |
| Duration of breastfeeding (months) | 950 | 8.9 | (0.8) | 8.8 | (0.7) | 8.9 | (0.9) |
| Discussed FP with provider4 | |||||||
| Never | 955 | 523 | (55%) | 258 | (67%) | 265 | (47%) |
| During ANC only | 955 | 106 | (11%) | 40 | (10%) | 66 | (12%) |
| During PNC only | 955 | 169 | (18%) | 51 | (13%) | 118 | (21%) |
| During ANC and PNC | 955 | 157 | (16%) | 40 | (10%) | 117 | (20%) |
|
| |||||||
| Clinical history | |||||||
|
| |||||||
| Ever diagnosed with STI | 934 | 33 | (4%) | 13 | (4%) | 20 | (4%) |
| HIV-infected4 | 940 | 81 | (8%) | 39 | (10%) | 42 | (7%) |
| Currently on ART (vs. not on ART)5 | 81 | 48 | (60%) | 23 | (58%) | 25 | (62%) |
FP=Family planning, ANC=antenatal care, PNC=postnatal care, IUD= intrauterine device, OCP = oral contraceptive pill, ART=antiretroviral therapy, STI=sexually transmitted infection. Adolescents are defined as age <20 years. Dual methods include condoms and another modern method. Percentages are weighted.
Effective contraception includes IUDs, implants, oral and injectable contraceptives, and tubal ligations
Number of observations with complete information included in model
Among women with current partners
Excludes 15 women with unknown HIV status
Among HIV-infected women
Among all 955 postpartum women, most (94%) reported no desire for children within the next two years and 80% resumed sexual activity by 9-months postpartum. Overall, 59% of all postpartum women (n=955) reported using modern contraception; the most common method was injectable (38%) (Figure 3). LARC was used by 12% of postpartum women. Less than 1% of women used TL, non-modern methods, or dual methods. Over half (56%) of postpartum women used effective contraception; among these women 61% used injectables, followed by implants (17%), OCs (11%), and IUDs (7%). Uptake of effective contraception varied regionally: Central (71%), Nairobi (65%), Eastern (62%), Western (59%), Nyanza (57%), Coast (55%), and Rift Valley (52%). Overall, 77% (n=733) of postpartum women had a need for FP. Among postpartum women with a need for FP, 30% were not currently using modern contraception.
Figure 3. Contraceptive methods used among Kenyan postpartum women attending maternal and child health clinics, 2013.
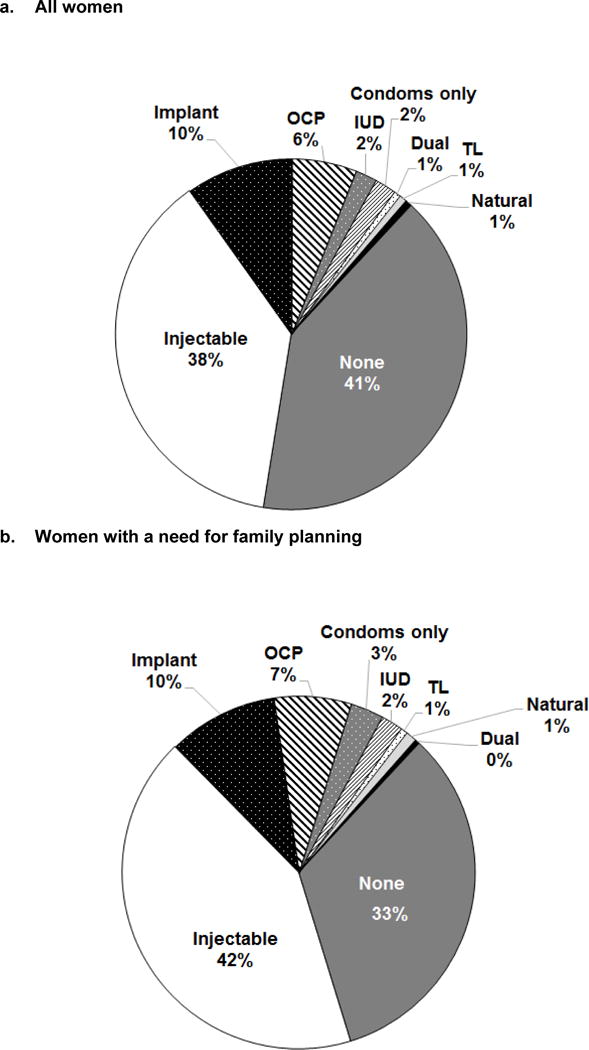
IUD= intrauterine device; OC = oral contraceptives; TL=tubal ligation. Dual methods include condoms and another effective method (injectables, implants, IUDs, OCs, and TL). Percentages are weighted. Percentages rounded up or down to nearest integer if >1%, which results in total exceeding 100%. Women with a need for FP had resumed sexual activity by 9 months postpartum and did not desire more children in the next 2 years.
Uptake of modern contraception and effective contraception was slightly lower among all postpartum women (62% and 59%, respectively) than the subset in women with a need for FP (70% and 67%, respectively) (Figures 4a and b). Injectable and LARC use were both similar among all women and women a need for FP, respectively. Prevalence of effective contraceptive use was also similar among HIV-infected and HIV-uninfected women (Figure 4a) (52% vs 60%, respectively; p=0.16).
Figure 4. Frequency of contraceptive use, by type and HIV status, among Kenyan postpartum women attending maternal and child health clinics, 2013.
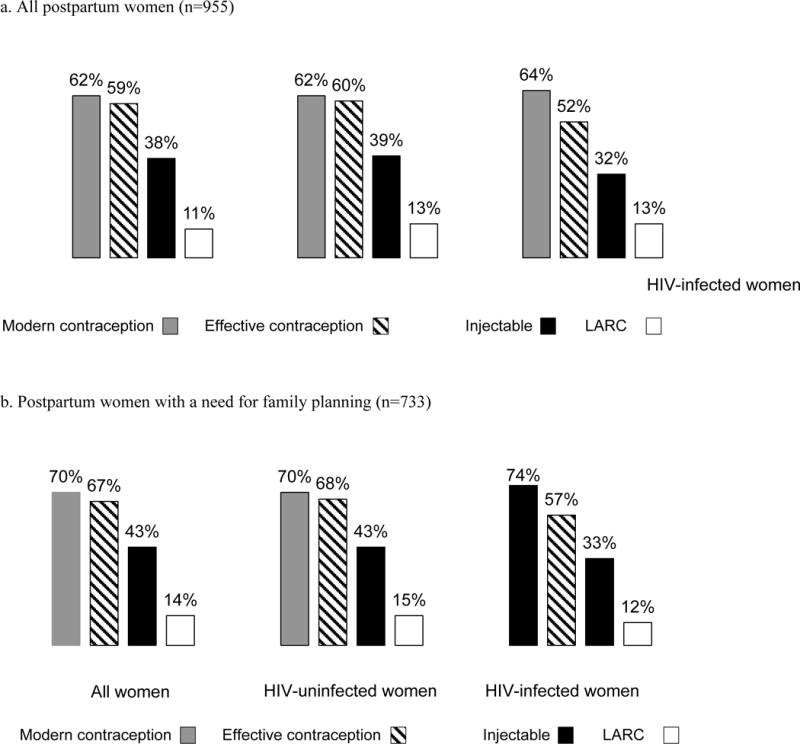
Women with unmet need reported resuming sexual activity after delivery and not desiring children within the next two years.
3.1 FP discussion
Less than half (44%) of all postpartum women reported discussing FP with a provider during ANC or PNC. Among these women, 11% discussed FP in ANC alone, 17% in PNC alone, and 16% in both ANC and PNC. Women who used effective contraception were more likely than women who did not use effective contraception to have discussed FP with a provider in PNC alone (21% vs. 12%, respectively) or in both ANC and PNC (21% vs. 9%, respectfully) (p<0.001 for both).
3.2 Correlates of effective contraceptive use
Among women with a need for FP, uptake of effective contraception was associated with higher education (Prevalence Ratio [PR] 1.06/year average increase, 95% Confidence Interval [CI] 1.03-1.08), household income ≥10,000 KSH/month (PR 1.21, 95% CI 1.08-1.37), and having two living parents (PR 1.22, 95% CI 1.06-1.40) (Table 2). Women ≤19 years were more likely to use effective contraception than older women. Effective contraceptive use was more common among women who delivered in a facility (PR 1.39, 95% CI: 1.18-1.64), and discussed FP in a healthcare setting compared to women who did not have any discussion about FP or discussed FP outside of a healthcare setting. While discussing FP in ANC alone was not associated with effective contraceptive use, discussing FP in PNC alone (PR 1.35, 95% CI 1.16-1.58) or in combination with ANC (PR 1.42, 95% CI: 1.21-1.67) were both correlates of use. Effective contraception was not significantly associated with the number of current or lifetime sexual partners, history of IPV, breastfeeding duration, HIV status, or partner’s HIV status (p>0.10). Several factors remained significantly associated with effective contraceptive use in a multivariate model that included education, age category, both parents being alive, facility delivery, ≥ 2 living children, ≥ 4 ANC visits, and discussed FP with provider in ANC and/or PNC (Table 2). Effective contraception was associated with higher education (Adjusted Prevalence Ratio [APR] 1.04/year increase, 95% CI 1.02-1.06, p<0.001). Delivering in a facility (APR 1.23, 95% CI 1.04-1.45, p=0.01) and discussing FP during PNC alone (APR 1.29, 95% CI 1.20-1.51, p=0.002) or during both ANC and PNC (APR=1.40, 95% CI 1.21-1.63, p<0.001) were also associated with effective contraceptive use. We conducted exploratory analyses among all postpartum women (with and without a need for FP) to determine whether uptake of postpartum contraception varied by FP method type or HIV status, and whether correlates of injectable use (vs. no effective contraception) differed from overall effective contraceptive use. Correlates of effective contraceptive use were similar to the primary analysis, with the exception that male partner ANC attendance (APR=1.17, 95% CI 1.02-1.34, p=0.02), having both parents alive (APR=1.16, 95% CI 1.02-1.33, p=0.03), and age 20–34 years (APR compared to age ≥35 1.44, 95% CI 1.04-2.00, p=0.03) were also significant in the adjusted model. Similarly, results from the injectable specific analysis and analysis stratified by HIV status were similar to the primary findings (data not shown).
Table 2.
Correlates of effective contraceptive use among Kenyan postpartum women attending maternal and child health clinics with a need for family planning, 2013. (n=733)1
|
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Weight Mean (SD) or Unweighted N (Weighted %) |
Weighted Poisson generalized linear models
|
|||||||
| Effective contraceptive use
|
||||||||
| N2 | No | Yes | p3 | Crude PR (95% CI) | p | Adjusted PR (95% CI)4 | p | |
|
|
||||||||
| Sociodemographic characteristics | ||||||||
|
| ||||||||
| Age category (years) | 733 | |||||||
| ≤19 | 16 (6%) | 30 (6%) | 0.006 | 1.33 (0.98-1.81) | 0.07 | 1.34 (0.99-1.81) | 0.06 | |
| 20-34 | 191 (78%) | 420 (86%) | 1.37 (1.09-1.74) | 0.008 | 1.27 (1.00-1.61) | 0.05 | ||
| ≥35 | 37 (15%) | 39 (8%) | Ref | |||||
| Completed education (per year increase) | 733 | 8.8 (4.3) | 10.2 (3.3) | <0.001 | 1.04 (1.02-1.05) | <0.001 | 1.03 (1.01-1.04) | 0.004 |
| Both parents alive (vs. one or both deceased) | 729 | 132 (54%) | 311 (64%) | 0.007 | 1.16 (1.04-1.30) | 0.01 | 1.08 (0.97-1.21) | 0.17 |
| Married | 733 | 233 (96%) | 466 (95%) | 0.9 | 0.98 (0.77-1.24) | 0.9 | ||
| Monthly household income ≥10,000 KSH | 606 | 37 (26%) | 118 (36%) | 0.05 | 1.14 (1.01-1.29) | 0.04 | ||
|
| ||||||||
| Relationships and sexual behavior | ||||||||
|
| ||||||||
| Relationship duration (per year increase)5 | 711 | 6.2 (5.3) | 5.2 (4.1) | 0.01 | 0.98 (0.97-1.00) | 0.01 | ||
| Partner age difference (per year older)5 | 646 | 5.8 (4.7) | 5.4 (4.8) | 0.4 | 0.99 (0.98-1.01) | 0.4 | ||
| ≥1 current sexual partners | 711 | 5 (2%) | 20 (4%) | 0.15 | 1.21 (0.98-1.48) | 0.07 | ||
| Number of lifetime sexual partners | 688 | 1.9 (1.0) | 1.9 (1.2) | 0.7 | 0.99 (0.95-1.04) | 0.8 | ||
| Intimate partner violence (within the past year) | 733 | 106 (43%) | 209 (42%) | 0.97 | 1.00 (0.90-1.11) | 0.97 | ||
| Partner HIV status known4 | 711 | 159 (67%) | 339 (71%) | 0.27 | 1.07 (0.95-1.20) | 0.28 | ||
| HIV-infected partner5,6 | 498 | 14 (8%) | 16 (5%) | 0.13 | 0.80 (0.58-1.12) | 0.2 | ||
|
| ||||||||
| Reproductive history | ||||||||
|
| ||||||||
| Gravidity ≥3 | 733 | 108 (44%) | 166 (34%) | 0.008 | 0.86 (0.77-0.97) | 0.01 | ||
| ≥2 living children | 733 | 168 (68%) | 281 (57%) | 0.004 | 0.86 (0.78-0.95) | 0.003 | ||
| ≥4 ANC visits | 689 | 115 (65%) | 268 (75%) | 0.017 | 1.18 (1.02-1.38) | 0.03 | 1.09 (0.94-1.27) | 0.2 |
| Partner attended ANC | 719 | 83 (35%) | 193 (41%) | 0.13 | 1.09 (0.98-1.21) | 0.12 | ||
| Facility delivery | 733 | 163 (67%) | 400 (81%) | <0.001 | 1.33 (1.15-1.55) | <0.001 | 1.26 (1.06-1.49) | 0.008 |
| Tried to become pregnant (last pregnancy) | 732 | 155 (64%) | 327 (68%) | 0.33 | 1.06 (0.94-1.18) | 0.34 | ||
| Duration of breastfeeding (per month increase) | 730 | 8.9 (0.5) | 8.9 (0.6) | 0.96 | 1.00 (0.92-1.10) | 0.96 | ||
| Discussed FP with provider during ANC or PNC (vs. neither) | 733 | 88 (35%) | 262 (53%) | <0.001 | 1.27 (1.14-1.41) | <0.001 | ||
| Discussed FP with provider | 733 | |||||||
| Never | 733 | 156 (65%) | 227 (47%) | <0.001 | ref | |||
| During ANC only | 733 | 28 (11%) | 58 (12%) | 1.14 (0.97-1.36) | 0.12 | 1.07 (0.89-1.28) | 0.5 | |
| During PNC only | 733 | 35 (14%) | 110 (23%) | 1.28 (1.13-1.45) | <0.001 | 1.25 (1.10-1.42) | 0.001 | |
| During ANC and PNC | 733 | 25 (10%) | 94 (19%) | 1.34 (1.18-1.52) | <0.001 | 1.33 (1.18-1.51) | <0.001 | |
|
| ||||||||
| Clinical history | ||||||||
|
| ||||||||
| Ever previously diagnosed with STI | 733 | 7 (3%) | 18 (4%) | 0.6 | 1.07 (0.83-1.38) | 0.6 | ||
| HIV-infected | 723 | 24 (9%) | 30 (6%) | 0.11 | 0.84 (0.66-1.07) | 0.15 | ||
| Currently on ART (vs. not on ART)7 | 54 | 14 (58%) | 17 (57%) | 0.96 | 0.99 (0.61 -1.61) | 0.96 | ||
PR=prevalence ratio, p=p-value, CI=confidence interval, FP=FP, ANC=antenatal care, PNC=postnatal care, IUD= intrauterine device, OCP = oral contraceptive pill, ART=antiretroviral therapy, STI=sexually transmitted infection. Dual methods include condoms and another modern method. Women with a need for FP had resumed sexual activity by 9-months postpartum and did not desire more children in the next 2 years. Percentages are weighted.
Effective contraception defined as intrauterine devices, implants, oral and injectable contraceptives, dual methods (condoms and hormonal method) or tubal ligation
Number of observations with complete information included in model
Chi-squared test for proportions and T-tests for continuous measures
Adjusted for education (completed years), age category, both parents being alive, number of ANC visits during last pregnancy and delivery at facility. Number of living children, gravidity and relationship duration were excluded due to collinearity with age. Income was excluded due to collinearity with education. Number of ANC visits during last pregnancy was excluded due to collinearity with facility delivery.
Among women with current male partners
Compared to women with HIV-uninfected male partners
Among HIV-infected women (n=81)
4.0 DISCUSSION
In this national survey of postpartum Kenyan women, we found that 56% were using effective contraception by 9-months postpartum, the majority (61%) of whom used injectable methods, and <1% used dual methods. The contraceptive prevalence rate (CPR) at 9-months postpartum in our study was similar to other recent studies of Kenyan women; however, use of LARC and permanent methods was lower and injectable use was higher in our study.[6, 9] Overall, 34% of postpartum women had an unmet need for FP.
We found that women who discussed FP during PNC, alone or in combination with FP discussion in ANC, were more likely to use effective contraception. A recent review of interventions to improve postpartum contraception found that interventions delivered after, rather than before, delivery are generally more successful but results are mixed.[20] Similarly, another observational study found no association between FP counseling in ANC and postpartum FP uptake.[21] Women in LMICs may be more receptive to FP discussions following delivery when there is a current or perceived future need for FP than during ANC.[20, 22] In our study, more than half of women reported never discussing FP within a healthcare setting, despite routinely attending ANC and PNC visits. Current Kenyan national guidelines include providing FP counseling throughout the continuum of care in ANC, intrapartum, and at each PNC visit; and as integrated service provision within HIV care.[23] Since infant immunization coverage is high in sub-Saharan Africa, with frequent provider interactions during these visits, strategies to promote FP counseling in the early postpartum period during infant immunization visits could be a useful strategy to reduce unmet need during this period.
In our study, higher maternal education and utilization of MCH services (≥4 ANC visits and facility delivery) were significantly associated with contraceptive use, consistent with other studies.[8–12, 24] Postpartum FP use in LMICs has also been associated with urban residence,[8] being married,[12] greater wealth and education,[10–12] achievement of desired family size and current fertility desire.[24] In contrast to our study, a study conducted in five LMICs found that younger postpartum women (<20 years old) were 24% more likely than women ≥30 years to have an unmet need for FP.[9] Together, these factors may be markers of empowerment and access to information that facilitate uptake of postpartum FP.
Over one-third of postpartum women in our study used injectables, and use was similar by HIV status. In response to findings from some, but not all, studies showing an association between depot medroxyprogesterone acetate (DMPA) and increased risk of HIV acquisition, [25–27] WHO recommended expanding the contraceptive method mix to include the full range of contraceptive methods (particularly implants and IUDs). Due to injectable widespread use in many regions with high HIV incidence, it is important to weigh potential HIV-related risks with potential benefits to avert maternal deaths via injectables.[28] Increasing implant use among postpartum women may help alleviate these HIV-related risks, and maintain MCH benefits. Implants have many characteristics that make them particularly well-suited for postpartum women, including the ability to be inserted immediately postpartum, breastfeeding compatibility, longer-term coverage, high effectiveness, and no need for re-dosing.[29] However, LARC use has previously been reported to be low (<4%) among postpartum women in sub-Saharan Africa.[9] Our data suggest implants are increasingly an acceptable FP method for postpartum women, with 10% using selecting this method, similar to the general population.[6] Thus, implants may increasing represent a larger proportion of the method mix to postpartum women who are offered them.
Our study had several strengths. We included adolescents (age 14-17) and unmarried postpartum women sampled nationally, restricted most analyses to women with a need for FP, and excluded FP methods with high failure rates. The study also had some limitations. One province was not included; however, it is sparsely populated and the sampling frame excluded small facilities; we did not measure contraceptive discontinuations or switching, and results may not be generalizable to women who do not attend PNC. Finally, we had limited power to assess correlates of contraceptive use among HIV-infected women.
Promoting FP during the postpartum period is a high-impact and cost-effective approach to improve MCH.[30] Strategies that better integrate patient-centered FP counseling and education within ANC and PNC as part of the routine package of MCH services, and provide comprehensive counseling to include methods with longer-term coverage could be effective approaches to meet postpartum FP needs. Further research to provide critical insights to enhance FP counseling approaches for postpartum women who are already accessible in the healthcare system, such as concerns about side effects and compatibility with breastfeeding, and data on method satisfaction and continuation rates among postpartum women, may help improve care.
Acknowledgments
We would like to acknowledge and thank our study participants and staff for their contributions to this project.
FUNDING
This publication was made possible by support from the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) through cooperative agreement [#U2GPS002047] from the U.S. Centers for Disease Control and Prevention (CDC), Division of Global HIV/TB (DGHT). JP and CJM were supported by the University of Washington STD/AIDS Research Training Fellowship (NIH NRSA T32AI007140), ALD was supported by a NIH career development award (NIAID K01 AI116298), and support for GJS includes a NIH K24 grant (HD054314).
Footnotes
DISCLOSURE OF INTERESTS
Conflicts of interest: There are no conflicts of interest.
CONTRIBUTION TO AUTHORSHIP
All authors contributed to the preparation of this manuscript (DA, JP, CJM, JK, JU, NO, AL, GJS, ALD). JK, CJM, AL, and GJS developed the idea for the study and assisted with study implementation. JK, CJM, and AL were involved in data collection activities. DA, JP, CJM, AL, GJS, ALD participated in drafting and revising the manuscript with input from co-authors; JK, JU, NO provided substantial revisions and intellectual content to the manuscript. JP analyzed the data and DA, JP, CJM, AL, and GJS, and ALD had full access to the data and take responsibility for the integrity and accuracy of the data. All authors (DA, JP, CJM, JK, JU, NO, AL, GJS, ALD) contributed to interpreting the data and approved the final version of the manuscript.
ETHICAL APPROVAL
All study procedures were approved by ethical review committees at the Kenya Medical Research Institute (KEMRI/RES/7/3/1, SSC No 2200), the University of Washington (IRB Application Number 41953), and the Associate Director for Science at the U.S. Centers for Disease Control and Prevention. Women provided written informed consent to participate.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the view of the U.S. Centers for Disease Control and Prevention.
References
- 1.United Nations. Transforming our world: the 2030 Agenda for Sustainable Development. 2015 [Google Scholar]
- 2.Brown W, Druce N, Bunting J, et al. Developing the “120 by 20” goal for the Global FP2020 Initiative. Stud Fam Plann. 2014;45:73–84. doi: 10.1111/j.1728-4465.2014.00377.x. [DOI] [PubMed] [Google Scholar]
- 3.DaVanzo J, Hale L, Razzaque A, Rahman M. Effects of interpregnancy interval and outcome of the preceding pregnancy on pregnancy outcomes in Matlab, Bangladesh. BJOG. 2007;114:1079–87. doi: 10.1111/j.1471-0528.2007.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rutstein S. Further evidence of the effects of preceding intervals on neonatal, infant, and under-five mortality and nutritional status in developing countries: evidence from the Demographic and Health Surveys, DHS Working Papers, Calverton, MD, USA. Macro International. 2008;(41) doi: 10.1016/j.ijgo.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Moore Z, Pfitzer A, Gubin R, Charurat E, Elliott L, Croft T. Missed opportunities for family planning: an analysis of pregnancy risk and contraceptive method use among postpartum women in 21 low- and bottom-income countries. Contraception. 2015;92:31–9. doi: 10.1016/j.contraception.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Kenya National Bureau of Statistics (KNBS) and ICF Macro. Kenya Demographic and Health Survey 2014. Rockville, Maryland: KNBS and ICF International; 2015. [Google Scholar]
- 7.Lutter CK, Daelmans BM, de Onis M, et al. Undernutrition, poor feeding practices, and low coverage of key nutrition interventions. Pediatrics. 2011;128:e1418–27. doi: 10.1542/peds.2011-1392. [DOI] [PubMed] [Google Scholar]
- 8.Mengesha ZB, Worku AG, Feleke SA. Contraceptive adoption in the extended postpartum period is low in Northwest Ethiopia. BMC Pregnancy Childbirth. 2015;15:160. doi: 10.1186/s12884-015-0598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasha O, Goudar SS, Patel A, et al. Postpartum contraceptive use and unmet need for family planning in five low-income countries. Reprod Health. 2015;12(Suppl 2):S11. doi: 10.1186/1742-4755-12-S2-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hounton S, Winfrey W, Barros AJ, Askew I. Patterns and trends of postpartum family planning in Ethiopia, Malawi, and Nigeria: evidence of missed opportunities for integration. Glob Health Action. 2015;8:29738. doi: 10.3402/gha.v8.29738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akinlo A, Bisiriyu A, Esimai O. DHS Working Papers. 92 Calverton, MD, USA: ICF International; 2013. Influence of Use of Maternal Health Care on Postpartum Contraception in Nigeria. [Google Scholar]
- 12.Do M, Hotchkiss D. Relationships between antenatal and postnatal care and post-partum modern contraceptive use: evidence from population surveys in Kenya and Zambia. BMC Health Serv Res. 2013;13:6. doi: 10.1186/1472-6963-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ronen K, McGrath CJ, Langat AC, et al. Gaps in Adolescent Engagement in Antenatal Care and Prevention of Mother-to-Child HIV Transmission Services in Kenya. J Acquir Immune Defic Syndr. 2017;74:30–7. doi: 10.1097/QAI.0000000000001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sherin KM, Sinacore JM, Li XQ, Zitter RE, Shakil A. HITS: a short domestic violence screening tool for use in a family practice setting. Family medicine. 1998;30:508–12. [PubMed] [Google Scholar]
- 15.Trussell J. Contraceptive failure in the United States. Contraception. 2011;83:397–404. doi: 10.1016/j.contraception.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization (WHO) Department of Reproductive Health and Research. Family planning: a global handbook for providers (2011 Update) Baltimore, MD; Geneva, Switzerland: 2011. [Google Scholar]
- 17.United States Centers for Disease Control and Prevention. Effectiveness of Family Planning Methods
- 18.Breslow NE. Generalized Linear Models: Checking Assumptions and Strengthening Conclusions. Statistica Applicata. 1996;8:23–41. [Google Scholar]
- 19.Lee J, Chia KS. Estimation of prevalence rate ratios for cross sectional data: an example in occupational epidemiology. Br J Ind Med. 1993;50:861–2. doi: 10.1136/oem.50.9.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cleland J, Shah IH, Daniele M. Interventions to Improve Postpartum Family Planning in Low- and bottom-Income Countries: Program Implications and Research Priorities. Stud Fam Plann. 2015;46:423–41. doi: 10.1111/j.1728-4465.2015.00041.x. [DOI] [PubMed] [Google Scholar]
- 21.Elul B, Delvaux T, Munyana E, et al. Pregnancy desires, and contraceptive knowledge and use among prevention of mother-to-child transmission clients in Rwanda. AIDS. 2009;23(Suppl 1):S19–26. doi: 10.1097/01.aids.0000363774.91376.dc. [DOI] [PubMed] [Google Scholar]
- 22.Ayiasi RM, Muhumuza C, Bukenya J, Orach CG. The effect of prenatal counselling on postpartum family planning use among early postpartum women in Masindi and Kiryandongo districts, Uganda. Pan Afr Med J. 2015;21:138. doi: 10.11604/pamj.2015.21.138.7026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Republic of Kenya Ministry of Health. National Family Planning Guidelines for Service Providers. Division of Reproductive Health. 2010 [Google Scholar]
- 24.Winfrey W, Kshitiz R. Use of Family Planning in the Postpartum Period. Rockville, Maryland, USA: ICF International; 2014. (DHS Comparative Report No 36). [Google Scholar]
- 25.Polis CB, Curtis KM, Hannaford PC, et al. An updated systematic review of epidemiological evidence on hormonal contraceptive methods and HIV acquisition in women. AIDS. 2016;30:2665–83. doi: 10.1097/QAD.0000000000001228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polis CB, Phillips SJ, Curtis KM, et al. Hormonal contraceptive methods and risk of HIV acquisition in women: a systematic review of epidemiological evidence. Contraception. 2014;90:360–90. doi: 10.1016/j.contraception.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Ralph LJ, McCoy SI, Shiu K, Padian NS. Hormonal contraceptive use and women’s risk of HIV acquisition: a meta-analysis of observational studies. Lancet Infect Dis. 2015;15:181–9. doi: 10.1016/S1473-3099(14)71052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butler AR, Smith JA, Polis CB, Gregson S, Stanton D, Hallett TB. Modelling the global competing risks of a potential interaction between injectable hormonal contraception and HIV risk. AIDS. 2013;27:105–13. doi: 10.1097/QAD.0b013e32835a5a52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medical eligibility criteria for contraceptive use – fifth edition 2015. Geneva: World Health Organization; 2015. http://www.who.int/reproductivehealth/publications/family_planning/Ex-Summ-MEC-5/en/, Accessed May 17, 2016. [PubMed] [Google Scholar]
- 30.Black RE, Levin C, Walker N, et al. Reproductive, maternal, newborn, and child health: key messages from Disease Control Priorities 3rd Edition. Lancet. 2016 doi: 10.1016/S0140-6736(16)00738-8. [DOI] [PubMed] [Google Scholar]


