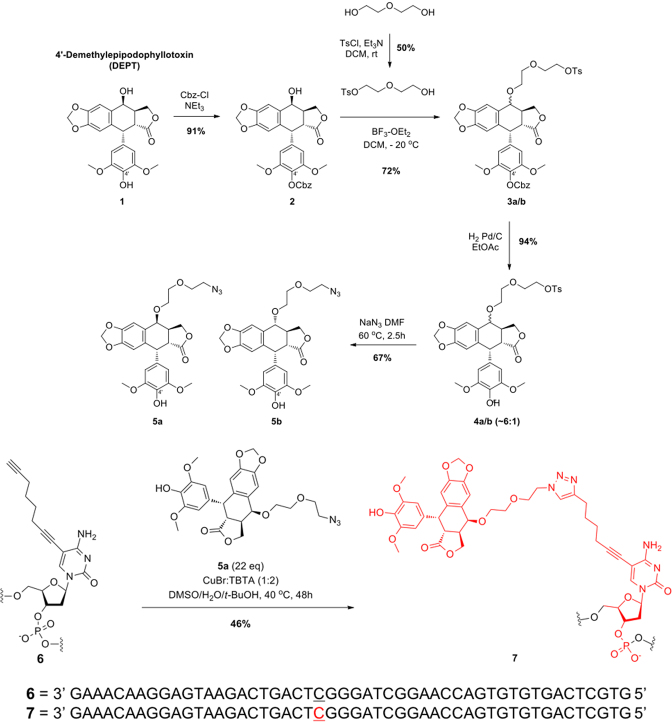
Figure 2.
Schematic of the synthesis of OTIs. The synthesis of OTI28 (7) is shown as an example. DEPT (1) was protected with carboxybenzyl (Cbz) at the 4′-OH using benzyl chloroformate and triethylamine in dichloromethane to yield 2. The 4-OH of 2 was reacted with monotosylated diethylene glycol using boron trifluoride etherate in dichloromethane at –20°C to generate 3a–b as a mixture of two epimers. Removal of the Cbz protecting group under hydrogenation reaction conditions with Pd/C in ethanol resulted in 4a–b. The tosyl group was displaced with sodium azide in dimethyl formamide at 60°C to generate 5a–b as a mixture of two epimers. The desired azide coupling partner 5a was purified as a single epimer by chiral chromatography. Copper-catalyzed click chemistry was used to couple 5a to oligonucleotide 6 (which included an alkyne-modified cytosine at position 28) to yield OTI28 (7).