Abstract
BACKGROUND
Obstructive sleep apnea (OSA) is associated with vascular endothelial dysfunction (VED) in otherwise healthy patients. The role of renin–angiotensin system (RAS) in the OSA induced VED is not well understood.
METHODS
Recently diagnosed OSA patients with very low cardiovascular disease (CVD) risk (Framingham score <5%) were studied at diagnosis and after 12 weeks of verified continuous positive airway pressure (CPAP) therapy. Participants underwent biopsy of gluteal subcutaneous tissue at baseline and after CPAP. Microcirculatory endothelial expression of angiotensin receptors type-1 (AT-1) and type-2 (AT-2) was measured in the subcutaneous tissue using quantitative confocal microscopy techniques. The ex-vivo effect of AT-1 receptor blockade (ARB) on endothelial superoxide production was also measured before and after CPAP treatment.
RESULTS
In OSA patients (n = 11), microcirculatory endothelial AT1 expression decreased from 873 (200) (fluorescence units) at baseline to 393 (59) units after 12 weeks of CPAP (P = 0.02). AT2 expression did not decrease significantly in these patients (479 (75) to 329 (58) post CPAP (P = 0.08)). The ex-vivo addition of the losartan to the microcirculatory endothelium resulted in decreased superoxide expression in the vascular walls from 14.2 (2.2) units to 4.2 (0.8) P < 0.001; while it had no effect on post-CPAP patient tissue (P = 0.64).
CONCLUSIONS
In OSA patients with no to minimal CVD risk, VED is associated with upregulation of AT-1 expression that is reversible with CPAP. Endothelial oxidative stress was reversible with ARB. RAS activation may play an important role in the development of early CVD risk in OSA patients.
Keywords: blood pressure, endothelial dysfunction, hypertension, nitric oxide, obstructive sleep apnea
Patients with obstructive sleep apnea (OSA) who otherwise have no apparent cardiovascular disease (CVD) manifest vascular endothelial dysfunction (VED) that is reversible with continuous positive airway pressure (CPAP).1–3 VED is the earliest predictor of subsequent development CVD in the general population.4,5 Therefore, understanding the mechanism of VED in OSA is critical to the understanding and treatment of the cardiovascular consequences of OSA.
Nitric oxide (NO) availability in the vascular endothelium is the critical determinant of endothelial function and vascular health.6,7 We recently developed a method for ex-vivo examination of the microcirculatory endothelium in OSA patients.1 Using this approach, we found decreased expression of NO and increased production of superoxide (O2−) in the microcirculatory endothelium that were reversible with CPAP. These studies support that dysfunction in endothelial NO synthase (eNOS) termed “eNOS uncoupling” occurs in OSA patients who are otherwise free of CVD.2 eNOS uncoupling occurs in environments with abundance of oxidants that can modify the function or structure of eNOS protein.8–10 Consequently, identification of the sources of endothelial oxidant stress is important for understanding the pathogenesis of this abnormality in the OSA patients’ endothelium. An important source of endothelial O2− overproduction is the NADPH oxidase system.11 In turn, the renin–angiotensin system (RAS) plays a role in NADPH oxidase activation in CVD12 with consequent O2− overproduction.12,13
Recent studies demonstrated that acute human exposure to intermittent hypoxia (IH) results in angiotensin II (Ang II) dependent activation of the NADPH system.14 Similarly, animal models of IH also demonstrate RAS activation and overexpression of angiotensin receptors type-1(AT-1) in association with VED.15,16 Other studies in OSA patients yielded conflicting results as far as the RAS activation and circulating Ang II levels in patients.17,18
We sought to study OSA patients who are otherwise free of CVD risk to better understand the profile of OSA-related VED. Based on previous studies, we hypothesized that OSA-related VED in this population is associated with early activation of RAS and endothelial O2− overproduction. In these patients, an evidence of increased angiotensin activity and involvement in the endothelial oxidant bursts would provide a targetable mechanistic pathway to address early cardiovascular risk in OSA patients who may be intolerant of CPAP.
METHODS
Participants
Patients with OSA.
Newly diagnosed patients were recruited from the Ohio State University Sleep Center within 2 weeks of their diagnostic polysomnography and prior to the initiation of CPAP therapy. Only patients with an apnea hypopnea index (AHI) >15 events per hour of sleep on the polysomnography were included. Low cardiovascular risk status was required in all participants and was defined by a Framingham risk Score <5%.19 In particular, exclusion criteria included any diagnosis or ongoing treatment of hypertension, hypercholesterolemia (total cholesterol >200 mg/dl regardless of age), diabetes, or smoking. None of the participants were on any prescribed medications; and supplements were discontinued at least a week prior to participation. The purpose of the restrictive inclusion criteria is to assure that VED in participants is due to OSA only and not to other subclinical cardiovascular risk factors.
Non-OSA participants.
We enrolled healthy participants with no OSA to serve as controls. While the study was designed to perform within subject comparison, measurements from the non-OSA participants were used for validation and sensitivity analyses. Non-OSA participants underwent either a polysomnography or home sleep test to rule out OSA. Participants met the same exclusion criteria as the OSA patients.
Procedures
OSA patients provided a baseline visit within 2 weeks of initial OSA diagnosis, prior to starting CPAP; and a conclusion visit after 12 weeks of CPAP therapy. Adherence to CPAP was verified with device download during the conclusion visit. Each visit included endothelial reactivity study and gluteal subcutaneous biopsy. Only patients who used CPAP more than 4.5 hours per night proceeded to complete the second visit with the biopsy and endothelial reactivity studies. Non-OSA volunteers underwent both procedures once. The protocol was approved by the OSU Institutional Review Board (protocol number 2009H0212). The study was registered in the National Clinical Trials database (NCT01027078).
Gluteal subcutaneous biopsy.
Incisional skin biopsy techniques were used to obtain 2–3 cm3 of subcutaneous tissue from the lateral upper gluteal region of participants. The biopsy tissue was immediately sectioned and a portion frozen in liquid nitrogen (N2) and kept in −80 °C for the confocal microscopy studies.
Endothelial reactivity studies.
Doppler ultrasound was used to measure flow-mediated dilation (FMD) of the brachial artery. Image acquisition was done with a linear array transducer (7 MHz frequency) and color spectral Doppler (GE Vivid 7). FMD measurements were performed according to published guidelines20 and our previously reported studies.1,2 Briefly, participants underwent the measurement between 7 and 9 AM prior to the biopsy procedure. All participants were instructed to abstain from caffeine for at least 12 hours prior to the measurements. None of the participants used tobacco, and all discontinued any supplements at least 1 week prior to participation.
Measurements
Quantification of Ang II receptors.
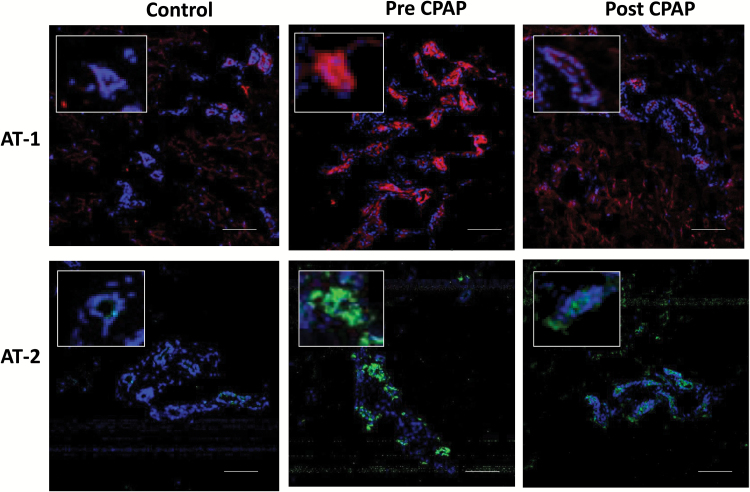
The expression of Ang II type 1 (AT1) and type 2 (AT2) receptors in the microcirculatory vascular endothelium was measured using quantitative confocal microscopy techniques. Subcutaneous tissue was immediately cryopreserved and later cryosectioned into 5 µM thick sections. The sections were fixed (4% paraformaldehyde) and then incubated with either anti-rabbit-AT1 and -AT2 (Novus Biologicals). Following incubation with the chosen primary antibodies (1:500 dilutions), the sections were incubated with specific anti-rabbit IgG (Alexa Fluor 488-conjugated) secondary antibodies (1:1000 dilution) and analyzed by Olympus Fluo-View 1000 confocal microscope (Olympus America, NY) with 20× objective and with the 405 nm and 488 nm excitations for Hoescht and green fluorescence respectively. For quantitation of angiotensin receptors, the respective fluorescence intensity was measured at the same laser setting for the green fluorophore and averaged per each condition. Figure 1 shows representative images of the AT1 and AT2 fluorescence in an OSA patient and a matched control.
Figure 1.
Detection of angiotensin receptors type 1 and 2 (AT-1 and AT-2) expression in the microcirculatory endothelium. Top: Representative confocal microscopy images of AT-1 fluorescence (red) in the frozen sections (8 µm) from one control and one OSA patient before and after treatment with CPAP. Bottom AT-2 fluorescence (green) in the same control and OSA patient. Magnification, ×20. Insets show higher magnifications of the endothelium/cells within squares. Bars: 100 µm. Abbreviations: AT, angiotensin receptors type; CPAP, continuous positive airway pressure; OSA, obstructive sleep apnea.
Effect of AT-1 blockade on O2− production in the microcirculatory endothelium.
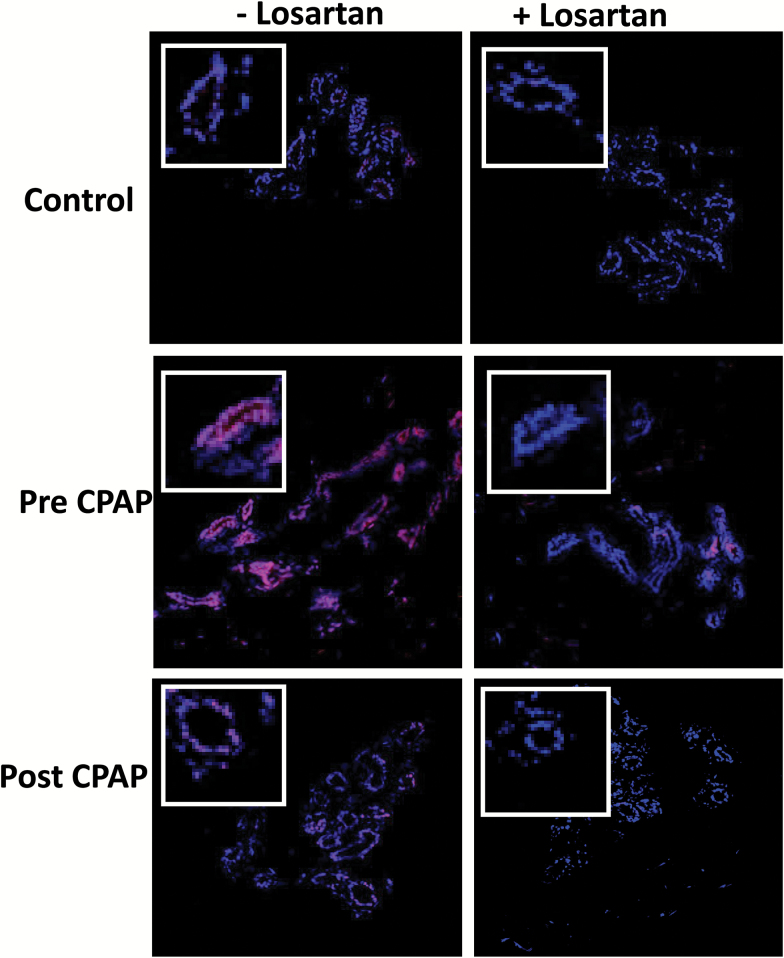
We determined O2−in situ production using dihydroethidium fluorescence microscopy techniques as we reported previously.2 Dihydroethidium is oxidized on reaction with O2− to ethidium, which binds to DNA in the nucleus and fluoresces red. Sections (5 µm) of the subcutaneous tissue were incubated with dihydroethidium (10 µM) along with Hoescht (1 µM) in dark for 30 minutes (at 37°C). The sections then were rinsed with tris-buffered saline for 5 minutes, fixed with paraformaldehyde, and then mounted with the antifade mounting medium, Fluoromount-G, by overlaying the coverslip. In initial experiments, the superoxide dismutase mimetic (MnTBAP) at 50 µM was added to the tissue sections and the resultant residual fluorescence values subtracted from the total fluorescence to determine the O2− derived signal. OSA patients’ tissue before and after CPAP was incubated with losartan (AT1 receptor blocker—ARB) to target the ang II effect on O2− in the tissue ex-vivo. Figure 2 shows representative images of these studies.
Figure 2.
Representative fluorescence photomicrographs of confocal optical sections of gluteal tissue biopsies from OSA patient before (pre) and after (post) CPAP and non-OSA volunteer (control), labeled with DHE with/without incubation of losartan. Each panel shows microvascular endothelial cell regions. Original magnification: ×20; bar, 100 µm, for all images. The intensity of DHE fluorescence measured from the corresponding sections (endothelium) of OSA patient (pre and post) CPAP and control tisuse samples, and after pretreatment of sections with the angiotensin inhibitor losartan (50 µM). Abbreviations: AT, angiotensin receptors type; CPAP, continuous positive airway pressure; DHE, dihydroethidium; OSA, obstructive sleep apnea.
NO production in the microcirculatory endothelium.
This measurement was performed as a confirmation that OSA patients with no to minimal CVD risk factors have underlying VED.2 We performed this measurement in addition to the FMD evaluation which is an indirect measurement of NO availability. Transverse sections (8 µm) were prepared from optimal cutting temperature-fixed tissue and incubated with the NO probe CuFL (500 µM) in the absence or presence of the NOS inhibitor: l-NG-Nitroarginine Methyl Ester (l-NAME) (1 mM). The specificity of CuFL fluorescence was confirmed by adding PTIO (NO scavenger, 50 µM) to the sections and confirmation of fluorescence quenching. The slides were viewed with Olympus Fluo-View 1000 confocal microscope at a magnification of ×20. The digital images were quantitatively analyzed for fluorescence intensities with the Olympus OIB software (FV10-ASW version 2.0). The CuFL technique has been widely applied for cellular and tissue measurements of NO.21,22 We have previously applied and validated the CuFL fluorescence along with the electron paramagnetic resonance measurement of NO.23 Inhibition of the observed CuFL derived fluorescence by NOS inhibition with l-NAME provides additional confirmation of specificity.
Design and analysis
For all microscopy studies and measurements, localization of the signal to the luminal walls of arterioles of >50 µ was performed by an observer blinded to the OSA or treatment status of the tissue source. All fluorescence and image quantification experiments were done simultaneously on all tissue in the same session. Intensity of the fluorescence was measured on a per pixel basis and this per pixel fluorescence intensity value was averaged for each contoured area of vascular endothelium. Measurements were obtained from multiple images of at least 3 sections.
FMD was measured according to published guidelines and our previously published work.1 Importantly, vessel images were obtained at baseline and at 60 seconds post release of occlusion. The images were de-identified and measured later by a blinded vascular imaging technologist.
Our primary hypothesis testing was the change in AT expression between pre-and post-CPAP. Based on animal studies evaluating the change in AT-1 expression with IH,16 we expected to see a similar effect in humans with CPAP (approximately 1 SD of change), and estimated a sample size of 10 OSA patients was required to achieve 80% power to detect this difference.
Patient characteristics are reported for OSA patients and non-OSA controls as mean and SD for continuous variables and count and percentage for sex. Median and interquartile range are also reported for baseline AHI. These characteristics were compared between groups using Wilcoxon rank–sum tests for continuous variables and Fisher’s exact test for sex.
NO expression and FMD were compared pre- vs. post-CPAP within patient using paired t-tests. Pearson’s correlation coefficients were calculated between baseline FMD and baseline AHI, age, and body mass index (BMI).
AT1 and AT2 expression levels were compared using linear mixed models, with a separate model each for AT1 and AT2. The models contained a random patient intercept to account for correlation between pre- and post-CPAP measures. The models also contained covariates for age and BMI to adjust for patient variability when making comparisons to controls. Linear estimates were constructed to test each hypothesis: pre CPAP vs. post CPAP within patient, pre CPAP vs. control, and post CPAP vs. control. The primary hypothesis testing involved within-patient comparisons using outcomes within the same OSA patients before and after verified treatment with CPAP. We accepted that the only change between the baseline visit and the conclusion visit was the elimination of OSA by CPAP. Testing hypotheses within-patient eliminates any effect of age, obesity, or other cardiovascular risk factors that are not addressed by the strict inclusion and exclusion criteria. For the losartan experiments, paired t-tests were used to compare mean O2− expression between samples with and without losartan incubation within each group/time point (pre CPAP, post CPAP, controls). All statistical tests were evaluated at the α = 0.05 significance level. All analyses were performed using SAS 9.4 (Cary, NC). The models controlled for age and BMI when making comparisons to the controls.
RESULTS
Characteristics of participants
Eleven OSA patients and 10 non-OSA volunteers participated in the study. Table 1 lists the characteristics of OSA patients. All OSA participants had total cholesterol <200 mg/dl as part of the inclusion criteria and were not on any medications. In addition to the characteristics listed in Table 1, AHI ranged from 12.6 to 120 events/min and desaturation index (4%) ranged from 6 to 90 episodes/h. OSA patients used CPAP (mean ± SD) 5.3 ± 1.2 hours per night, and had a post treatment AHI of 2.9 ± 2.6 events/hour on device download during the 12 week visit. Pre-CPAP weight vs. post-CPAP weight was unchanged.
Table 1.
Baseline patient characteristics
| Characteristic | OSA patients (n = 11) | Controls (n = 10) | P valuea |
|---|---|---|---|
| Age | 40.5 (12.6) | 36.8 (9.2) | 0.51 |
| BMI | 36.0 (8.1) | 30.4 (3.8) | 0.07 |
| Sex, male | 11 (100%) | 3 (30%) | 0.001 |
| SBP | 134 (13) | 127 (18) | 0.37 |
| DBP | 83 (4) | 79 (8) | 0.14 |
| ESS | 13.4 (5.1), n = 9 | 10.1 (4.8), n = 7 | 0.30 |
| Oxygen desaturation nadir | 80 (6), n = 10 | 91 (3), n = 8 | 0.004 |
| AHI; mean (SD); median (IQR, range) | 35 (27); 28 (17–46, 11–100) | 1.4 (1.7); 0.8 (0.4–1.5, 0–4.5), n = 9 | 0.002 |
Mean (SD) or n (%). Abbreviations: AHI, apnea hypopnea index; BMI, body mass index; DBP, diastolic blood pressure; ESS, Epworth sleepiness scale; IQR, interquartile range; SBP, systolic blood pressure.
aWilcoxon rank–sum tests except for sex (Fisher’s exact test).
There were no significant differences in age, BMI, blood pressure, or lipid profile between the 2 groups. However, given the imbalance in BMI, adjustment to BMI was performed in the 2 main comparisons in AT1 and AT2 expression between OSA pre-CPAP patients and the non-OSA group.
Endothelial function and NO availability.
In OSA patients (n = 11), baseline NO expression in the microcirculatory endothelium was 1.6 (0.9) fluorescence units and increased after 12 weeks of CPAP to 6.6 (1.6) P < 0.001.
Similarly, FMD was 4.59 (2.29) % at baseline in OSA patients (n = 8) and increased to 6.71 (3.17) %. In non-OSA controls (n = 9), FMD was 7.06 (3.83) %. Baseline FMD correlated negatively with the baseline AHI in patients (r = −0.72, P = 0.001). There was no correlation between FMD and age, or BMI in the OSA patients.
These data confirmed that OSA patients with low to no CVD risk status have baseline subclinical VED.
Expression of AT receptors in the microcirculatory endothelium of OSA patients
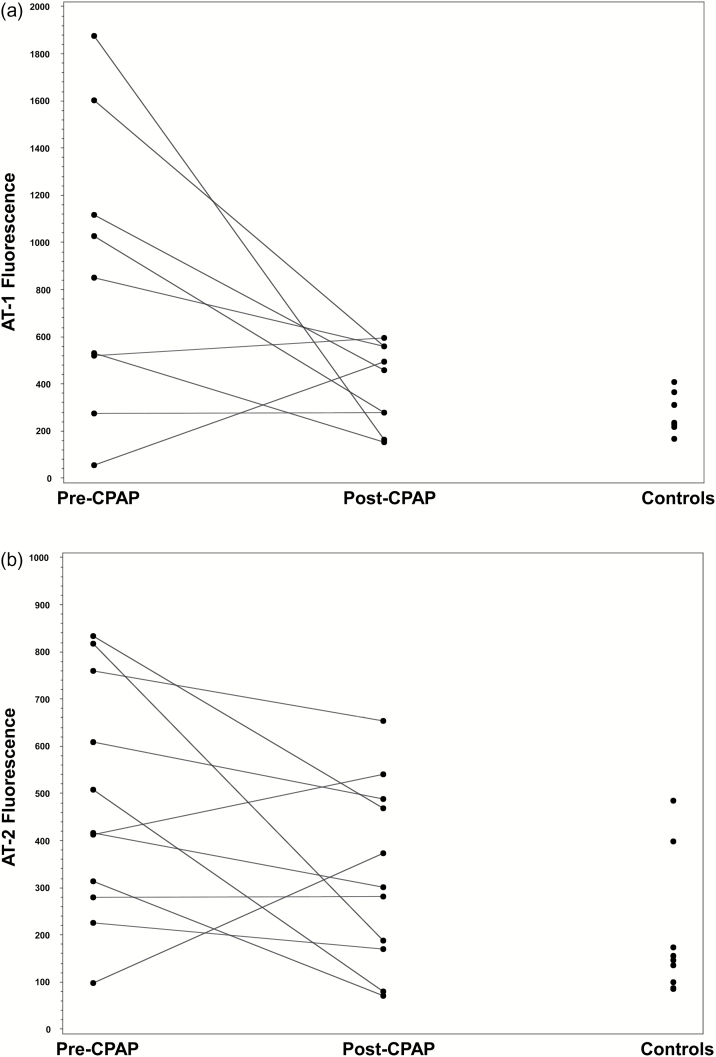
AT1 decreased from 873 (200) (fluorescence units) pre CPAP (n = 9) to 393 (59) units post CPAP (p = 0.02). AT-1 expression was 262 (32) fluorescence units in the control group (n = 8) and was significantly different from the pre-CPAP group (P = 0.02) but not significantly different from post CPAP (P = 0.74) (Figures 1 and 3a).
Figure 3.
Microvascular angiotensin receptors (type 1 and 2) expression in OSA patients and controls. (a) AT1 expression. (b) AT2 expression. Abbreviations: AT, angiotensin receptors type; CPAP, continuous positive airway pressure.
AT2 expression decreased in OSA patients (n = 11) with CPAP from 479 (75) to 329 (58) P = 0.08. Controls (n = 9) had an expression of 196 (48) units and were significantly different from pre-CPAP (P = 0.009) and not from post CPAP (Figures 1 and 3b).
Effect of ARB on endothelial O2− in OSA.
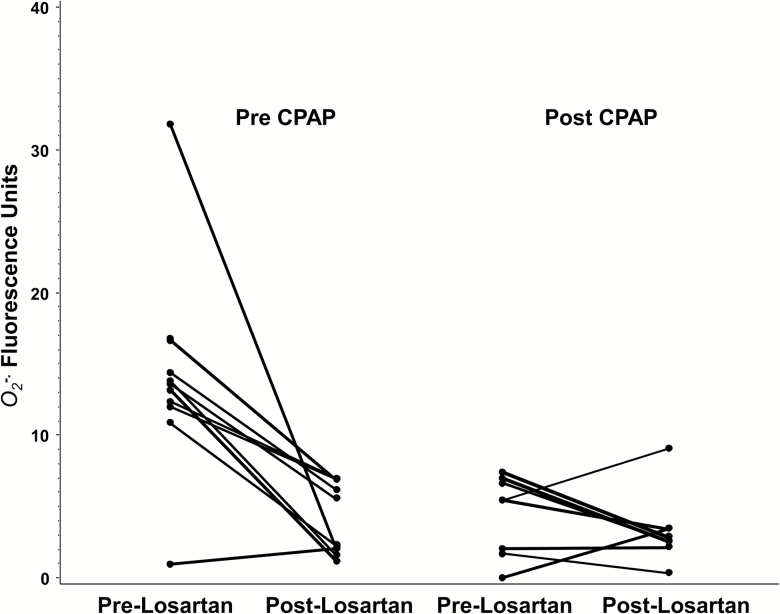
We analyzed the effect of incubation with losartan on the microvascular endothelial O2−ex-vivo using the confocal microscopy techniques described above. In pre-CPAP tissue (n = 11), incubation with losartan decreased O2− expression from 14.2 (2.2) units to 4.2 (0.8) P <0.001; while it had no effect on post-CPAP patient tissue 4.4 (1.0) to 3.5 (0.8) P = 0.64 (Figure 4). In a group of 3 controls, there was no change in O2− with losartan 2.4 (1.6) to 3.1 (0.9) P = 0.85 (data not depicted in Figure 4).
Figure 4.
Ex-vivo effect of losartan on endothelial superoxide expression in OSA patients before and after CPAP therapy. Abbreviations: CPAP, continuous positive airway pressure; OSA, obstructive sleep apnea.
DISCUSSION
In this study, we measured for the first time in humans with OSA the expression of angiotensin receptors in the microcirculatory vascular walls. We targeted OSA patients who are free of CVD risk in order to characterize the pathways underlying VED that would be attributable only to OSA in the absence of other cardiovascular risk factors in these individuals. Our findings confirm the role of the RAS activation in the preclinical cardiovascular consequences of OSA. In addition, the study demonstrated that ARB decreased the expression of superoxide in the vascular endothelium ex-vivo.
Previous studies have examined the RAS pathway in the pathogenesis of vascular disease in OSA patients and yielded mixed results.18,24,25 In an animal model of IH, Marcus et al. reported a role for AT-1 overexpression in IH-related VED.15 Similarly, Pialoux et al. reported that short-term IH exposure in healthy adults was associated with oxidative response that could be abolished with ARB.14 The current study makes the distinct contribution of demonstrating the upregulation of AT receptors in the peripheral microcirculation of OSA patients who are free of CVD risk factors. In addition, the ex-vivo effect of ARB, further supports a role for AT-1 in the OSA-related endothelial superoxide overproduction.
The cardiovascular effects of AT1 and AT2 and their interaction with Ang II in CVD are complex.26 Chronic AT-1 blockade attenuates Ang II-induced vasoconstriction via AT-2 NO dependent pathway.27 Furthermore, AT-2 expression is upregulated in the resistance arteries of diabetic hypertensive patients who were treated with AT-1 blockade and contributed to an Ang II-induced vasodilatory response.28 Our findings of more significant decrease in AT-1 than AT-2 expression with CPAP may support a similar role for AT-1 in the pathogenesis of VED in OSA patients. However, further studies are needed to elucidate the interactions between the AT-1 and AT-2 in the pathogenesis of CVD in OSA patients and the effect of therapy.
A possible consequence of RAS activation is the upregulation of NADPH oxidase is a one likely effect of Ang II on the endothelium.12 We previously found that OSA patients with no CVD risk factors have eNOS uncoupling which requires an environment of superoxide overproduction. The findings of this study of a relationship between AT-1 blockade and decreased superoxide expression may provide a potential mechanism for the eNOS uncoupling and VED in OSA patients. While the use of ARB for treatment of hypertension OSA patients is effective,29 it is unclear whether such approach would be an appropriate intervention to modify the early subclinical cardiovascular risk in OSA patients. Nevertheless, given that adherence to long-term therapy with CPAP is limited,30,31 the need for supplementary pharmacological intervention targeting CVD risk in OSA patients is likely justified.
This study as well as others provide evidence that OSA is associated with subclinical cardiovascular risk status. Nevertheless, recent trials have demonstrated that treatment of OSA does not decrease cardiovascular events in patients with advanced CVD.31 Reconciling these seemingly contradictory observations requires better understanding of the mechanism of CVD in OSA. One speculation is that OSA patients who already have established advanced CVD no longer can realize a relatively small incremental cardiovascular benefit of eliminating OSA. Another possibility is that current treatment of OSA with CPAP is simply not adequate to reverse the cardiovascular consequences fully.
Limitations and technical considerations
This study utilized quantitative confocal microscopy techniques to test the primary hypotheses of this research. Other methods for quantifying O2− such as electron paramagnetic resonance are more specific but less sensitive. However, the limited amount of available tissue in these human studies, precluded its application at this time.23,32 This approach allows the immediate preservation of microcirculatory vessels within the biopsy tissue no later than few minutes after the biopsy procedure. In addition, being able to localize the superoxide, NO, and AT receptors directly to the endothelial layer of the microcirculatory vessels enhanced the sensitivity and specificity of the measurements.
An important limitation that should be addressed in subsequent studies is the lack of a direct measurement of RAS activation. Only participants with very low cardiovascular risk status were included in this study. Therefore, the VED observed in OSA participants before treatment was attributable only to OSA. Furthermore, the primary comparisons were within subject before and after treatment eliminating any confounding effects of weight, age, or unidentified cardiovascular risk on the findings.
FUNDING
This research was supported by a grant from NIH- NHLBI (R56HL127079-01A1) and by the National Center for Research Resources (NCRR) award (ULRR025755).
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1. Patt BT, Jarjoura D, Haddad DN, Sen CK, Roy S, Flavahan NA, Khayat RN. Endothelial dysfunction in the microcirculation of patients with obstructive sleep apnea. Am J Respir Crit Care Med 2010; 182:1540–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Varadharaj S, Porter K, Pleister A, Wannemacher J, Sow A, Jarjoura D, Zweier JL, Khayat RN. Endothelial nitric oxide synthase uncoupling: a novel pathway in OSA induced vascular endothelial dysfunction. Respir Physiol Neurobiol 2015; 207:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med 2004; 169:348–353. [DOI] [PubMed] [Google Scholar]
- 4. Clarkson P, Celermajer DS, Powe AJ, Donald AE, Henry RM, Deanfield JE. Endothelium-dependent dilatation is impaired in young healthy subjects with a family history of premature coronary disease. Circulation 1997; 96:3378–3383. [DOI] [PubMed] [Google Scholar]
- 5. Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 2000; 101:948–954. [DOI] [PubMed] [Google Scholar]
- 6. Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA 1987; 84:9265–9269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3’:5’-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci USA 1977; 74:3203–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bouloumié A, Bauersachs J, Linz W, Schölkens BA, Wiemer G, Fleming I, Busse R. Endothelial dysfunction coincides with an enhanced nitric oxide synthase expression and superoxide anion production. Hypertension 1997; 30:934–941. [DOI] [PubMed] [Google Scholar]
- 9. Cosentino F, Hishikawa K, Katusic ZS, Lüscher TF. High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation 1997; 96:25–28. [DOI] [PubMed] [Google Scholar]
- 10. Bauersachs J, Bouloumié A, Fraccarollo D, Hu K, Busse R, Ertl G. Endothelial dysfunction in chronic myocardial infarction despite increased vascular endothelial nitric oxide synthase and soluble guanylate cyclase expression: role of enhanced vascular superoxide production. Circulation 1999; 100:292–298. [DOI] [PubMed] [Google Scholar]
- 11. Berry C, Hamilton CA, Brosnan MJ, Magill FG, Berg GA, McMurray JJ, Dominiczak AF. Investigation into the sources of superoxide in human blood vessels: angiotensin II increases superoxide production in human internal mammary arteries. Circulation 2000; 101:2206–2212. [DOI] [PubMed] [Google Scholar]
- 12. Rajagopalan S, Kurz S, Münzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 1996; 97:1916–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hanna IR, Taniyama Y, Szöcs K, Rocic P, Griendling KK. NAD(P)H oxidase-derived reactive oxygen species as mediators of angiotensin II signaling. Antioxid Redox Signal 2002; 4:899–914. [DOI] [PubMed] [Google Scholar]
- 14. Pialoux V, Foster GE, Ahmed SB, Beaudin AE, Hanly PJ, Poulin MJ. Losartan abolishes oxidative stress induced by intermittent hypoxia in humans. J Physiol 2011; 589:5529–5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marcus NJ, Philippi NR, Bird CE, Li YL, Schultz HD, Morgan BJ. Effect of AT1 receptor blockade on intermittent hypoxia-induced endothelial dysfunction. Respir Physiol Neurobiol 2012; 183:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marcus NJ, Li YL, Bird CE, Schultz HD, Morgan BJ. Chronic intermittent hypoxia augments chemoreflex control of sympathetic activity: role of the angiotensin II type 1 receptor. Respir Physiol Neurobiol 2010; 171:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Møller DS, Lind P, Strunge B, Pedersen EB. Abnormal vasoactive hormones and 24-hour blood pressure in obstructive sleep apnea. Am J Hypertens 2003; 16:274–280. [DOI] [PubMed] [Google Scholar]
- 18. Lykouras D, Theodoropoulos K, Sampsonas F, Lagiou O, Lykouras M, Spiropoulou A, Flordellis C, Alexandrides T, Karkoulias K, Spiropoulos K. The impact of obstructive sleep apnea syndrome on renin and aldosterone. Eur Rev Med Pharmacol Sci 2015; 19:4164–4170. [PubMed] [Google Scholar]
- 19. Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998; 97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 20. Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R; International Brachial Artery Reactivity Task Force . Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002; 39:257–265. [DOI] [PubMed] [Google Scholar]
- 21. Schreiber F, Beutler M, Enning D, Lamprecht-Grandio M, Zafra O, González-Pastor JE, de Beer D. The role of nitric-oxide-synthase-derived nitric oxide in multicellular traits of Bacillus subtilis 3610: biofilm formation, swarming, and dispersal. BMC Microbiol 2011; 11:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Efremova LV, Alekseeva AY, Konkova MS, Kostyuk SV, Ershova ES, Smirnova TD, Konorova IL, Veiko NN. Extracellular DNA affects NO content in human endothelial cells. Bull Exp Biol Med 2010; 149:196–200. [DOI] [PubMed] [Google Scholar]
- 23. Yang C, Talukder MA, Varadharaj S, Velayutham M, Zweier JL. Early ischaemic preconditioning requires Akt- and PKA-mediated activation of eNOS via serine1176 phosphorylation. Cardiovasc Res 2013; 97:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zalucky AA, Nicholl DD, Hanly PJ, Poulin MJ, Turin TC, Walji S, Handley GB, Raneri JK, Sola DY, Ahmed SB. Nocturnal hypoxemia severity and renin-angiotensin system activity in obstructive sleep apnea. Am J Respir Crit Care Med 2015; 192:873–880. [DOI] [PubMed] [Google Scholar]
- 25. Nicholl DD, Hanly PJ, Poulin MJ, Handley GB, Hemmelgarn BR, Sola DY, Ahmed SB. Evaluation of continuous positive airway pressure therapy on renin-angiotensin system activity in obstructive sleep apnea. Am J Respir Crit Care Med 2014; 190:572–580. [DOI] [PubMed] [Google Scholar]
- 26. Cosentino F, Savoia C, De Paolis P, Francia P, Russo A, Maffei A, Venturelli V, Schiavoni M, Lembo G, Volpe M. Angiotensin II type 2 receptors contribute to vascular responses in spontaneously hypertensive rats treated with angiotensin II type 1 receptor antagonists. Am J Hypertens 2005; 18:493–499. [DOI] [PubMed] [Google Scholar]
- 27. Savoia C, Ebrahimian T, He Y, Gratton JP, Schiffrin EL, Touyz RM. Angiotensin II/AT2 receptor-induced vasodilation in stroke-prone spontaneously hypertensive rats involves nitric oxide and cGMP-dependent protein kinase. J Hypertens 2006; 24:2417–2422. [DOI] [PubMed] [Google Scholar]
- 28. Savoia C, Touyz RM, Volpe M, Schiffrin EL. Angiotensin type 2 receptor in resistance arteries of type 2 diabetic hypertensive patients. Hypertension 2007; 49:341–346. [DOI] [PubMed] [Google Scholar]
- 29. Thunström E, Manhem K, Rosengren A, Peker Y. Blood pressure response to losartan and continuous positive airway pressure in hypertension and obstructive sleep apnea. Am J Respir Crit Care Med 2016; 193:310–320. [DOI] [PubMed] [Google Scholar]
- 30. Kushida CA, Nichols DA, Holmes TH, Quan SF, Walsh JK, Gottlieb DJ, Simon RD Jr, Guilleminault C, White DP, Goodwin JL, Schweitzer PK, Leary EB, Hyde PR, Hirshkowitz M, Green S, McEvoy LK, Chan C, Gevins A, Kay GG, Bloch DA, Crabtree T, Dement WC. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: The Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep 2012; 35:1593–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, Mediano O, Chen R, Drager LF, Liu Z, Chen G, Du B, McArdle N, Mukherjee S, Tripathi M, Billot L, Li Q, Lorenzi-Filho G, Barbe F, Redline S, Wang J, Arima H, Neal B, White DP, Grunstein RR, Zhong N, Anderson CS; SAVE Investigators and Coordinators . CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375:919–931. [DOI] [PubMed] [Google Scholar]
- 32. Lim MH, Xu D, Lippard SJ. Visualization of nitric oxide in living cells by a copper-based fluorescent probe. Nat Chem Biol 2006; 2:375–380. [DOI] [PubMed] [Google Scholar]