Abstract
BACKGROUND
Glomerular hyperfiltration may contribute to the high incidence of renal disease in Obese African Americans essential hypertensive (ObAAEH) patients, but the precise mechanisms responsible for renal injury have not been elucidated. Mitochondria are important determinants of renal injury in hypertension, and increased levels of mitochondrial DNA (mtDNA) in the urine may indicate renal mitochondrial injury. We hypothesized that urine mtDNA copy numbers would be higher in ObAAEH compared to Caucasian essential hypertensive (CEH) patients.
METHODS
We prospectively measured systemic, renal vein (RV), inferior vena cava (IVC), and urinary copy number of the mtDNA genes COX3 and ND1 by quantitative-PCR in CEH and ObAAEH patients during constant sodium intake and antihypertensive regimens, and compared them with healthy volunteers (HV) (n = 23 each).
RESULTS
Blood pressure was similarly elevated in CEH and ObAAEH, while glomerular filtration rate (GFR) was higher and age lower in ObAAEH. Urinary (but not plasma) COX3 and ND1 were higher in CEH compared to HV, and further elevated in ObAAEH patients. COX3 and ND1 renal gradients (RV–IVC) were higher in ObAAEH vs. CEH, and their urinary levels directly correlated with GFR. In multivariate analysis, GFR remained the only predictor of elevated urinary COX3 and ND1 levels.
CONCLUSIONS
Urinary fragments of the mitochondrial genome are elevated in ObAAEH patients and correlate with glomerular hyperfiltration. A positive gradient across the kidney in ObAAEH suggests selective renal release. These results are consistent with mitochondrial injury that may aggravate renal damage and accelerate hypertension-related morbidity/mortality rates in ObAAEH.
Keywords: African American, blood pressure, hyperfiltration, hypertension, mitochondria, obesity
The prevalence of hypertension in African Americans is among the highest in the world, reaching an alarming 44.9% and 46.1% in men and women, respectively.1,2 Obese African American essential hypertensive (ObAAEH) patients have an increased risk for developing chronic kidney disease and reach end-stage renal disease earlier than their Caucasian EH (CEH) counterparts.3 Therefore, there is a pressing need to elucidate the mechanisms implicated in hypertension-induced renal damage in this ethnic group.
Previous studies indicate that alterations of intrarenal hemodynamics may contribute to the higher incidence of renal injury in ObAAEH. Renal blood flow, glomerular filtration rate (GFR), and urinary albumin levels are elevated in ObAAEH and further increase in response to norepinephrine, suggesting glomerular hyperfiltration.4 Furthermore, ObAAEH have impaired GFR autoregulation and glomerular hyperfiltration in response to increased dietary sodium intake.5 In agreement, we have previously shown that GFR is higher in ObAAEH compared to CEH, associated with increased oxidative stress and renal medullary hypoxia.6 However, the exact molecular mechanisms underlying renal hyperfiltration in ObAAEH remain to be elucidated.
Mitochondria are present in large amounts in renal cells to satisfy the high energy demand of the kidney.7 These organelles are primarily responsible for ATP production, but also regulate cellular oxidative stress, proliferation, apoptosis, and calcium signaling.8,9 Thus, mitochondrial injury may compromise renal cell function.10 Experimental evidence suggests that mitochondrial damage is implicated in the pathogenesis of hypertension-induced renal injury,11 but human data are limited partly because it requires a renal biopsy, which is unlikely to be performed in uncomplicated hypertensive patients.
Increased levels of mitochondrial DNA (mtDNA) in the urine has emerged as a noninvasive and clinically useful index of renal mitochondrial dysfunction. Urinary mtDNA is elevated in patients with acute kidney injury and is associated with progression in its severity.12,13 Likewise, we have recently shown that elevated urinary mtDNA copy numbers in hypertensive patients correlated with markers of renal injury and dysfunction.14 However, whether disruption of mitochondrial integrity in the context of glomerular hyperfiltration may be associated with detectable release of mtDNA into the urine of ObAAEH has never been explored. Therefore, this study tested the hypothesis that urine mtDNA copy numbers originating in the kidneys would be higher in ObAAEH compared to CEH patients.
METHODS
Patient population
Nondiabetic CEH and ObAAEH patients (n = 23 in each group) from the Mayo Clinic (Rochester, MN), University of Mississippi (Jackson, MS), or University of Alabama (Birmingham, AL) were prospectively enrolled after providing written informed consent and the approval of the Institutional Review Board of the Mayo Clinic. Twenty-three consenting healthy volunteers (HV) served as controls (Mayo Clinic Biobank).
Inclusion criteria for hypertensive patients included blood pressure ≥140/90 mm Hg, previous diagnosis of hypertension, and/or current use of antihypertensive medications.
All CEH and ObAAEH patients participated in this study during a 3-day inpatient protocol in the Clinical Research Unit of St Mary’s Hospital, Rochester, MN. Dietary sodium intake was maintained at a constant level (150 mEq/d) for 3 days and patients continued with any prescribed angiotensin converting enzyme inhibitors or angiotensin receptor blockers at usual recommended daily doses. Patients with systolic blood pressure ≥180 mm Hg despite antihypertensive therapy, or serum creatinine ≥2.5 mg/dl, were excluded, as were those with a history of major cardiovascular events within the last 6 months, pregnancy, or kidney transplant.
HV were enrolled by using the following inclusion criteria: blood pressure ≤130/80 mm Hg, no history of hypertension or cardiovascular disease, and not taking antihypertensives, lipid-lowering drugs, or any medication listed in the algorithms from electronic Medical Records and Genomics.15
Clinical data collection and laboratory measurements
On day 1, demographics and laboratory data including age, gender, body mass index, systolic, diastolic, and mean arterial pressure were collected. Total cholesterol, low-density lipoprotein, and serum creatinine were measured by standard procedures.
Estimated GFR was calculated using the chronic kidney disease epidemiology collaboration (CKD-EPI) formula.16 In addition, measured GFR was quantified in all hypertensive patients (ObAAEH and CEH) by iothalamate clearance (iothalamate meglumine; Conray, www.imaging-.mallinckrodt.com).6,17 Urine samples were collected for 24-hour total protein levels in CEH and ObAAEH, whereas spot urine samples were obtained from HV. Samples were centrifuged, and the supernatant stored immediately at −80 °C until measurement to prevent catalytic degradation. Urinary protein, urinary sodium, and urinary creatinine levels were measured by standard procedures. Filtered sodium was calculated as the product of serum sodium and measured GFR and expressed as mEq/day. Fractional excretion of sodium was calculated with the following formula: (urinary sodium × plasma creatinine)/(plasma sodium × urinary creatinine) × 100, and total absolute amount of sodium reabsorbed as: (GFR × [plasma sodium]) − (urine flow rate × [urine sodium]).
On day 3, a catheter was placed via the femoral or internal jugular vein of CEH and ObAAEH patients and 120 ml of blood obtained from both the right and left renal veins (RVs) and the inferior vena cava (IVC).18,19 In HV, peripheral (antecubital) blood samples were collected.
Plasma and urine mtDNA
Plasma and urinary levels of the mitochondria encoded cytochrome-c oxidase-3 (COX3) and nicotinamide adenine dinucleotide dehydrogenase subunit-1 (ND1) were measured by quantitative real-time PCR, as previously described.14 In brief, plasma (200 µl) and urine (1.75 ml) mtDNA were isolated using DNA isolation kits from Qiagen, Venlo, Netherlands (Cat# 51104) and Norgen Biotek, Ontario, Canada (Cat# 18100), respectively. Isolated mtDNA was then eluted in the total volume of elution buffer (150 µl), DNA concentrations measured by a Spectrophotometer (NanoDrop), and 7.2 ng/sample added into PCR reactions. Primers and TaqMan copy number assays were purchased from Life Technology (Life Technology, Carlsbad, CA, COX3: forward 5ʹ-AGGCATCACCCCGCTAAATC-3ʹ and reverse 5ʹ-GGTGAGCTCAGGTGATTGATACTC-3ʹ and ND1: forward 5ʹ-AGTCACCCTAGCCATCATTCTACT-3ʹ and reverse 5ʹ-GGAGTAATCAGAGGTGTTCTTGTGT-3ʹ. Absolute COX3 and ND1 copy numbers were calculated using plasmid constructs (Blue Heron BioTechnology, Bothell, WA, NC0129201 and OriGene, Rockville, MD, SC101172, respectively) on Applied Biosystems ViiA7 Real-Time PCR systems. To identify mitochondrial-specific cellular damage, mtDNA copy numbers were corrected to the nuclear control gene RNAse-P (Invitrogen, Cat# 4403326) using a human genomic DNA for the standard curve (Invitrogen, Cat# 360486) and urinary creatinine (Arbor assays, Cat# K002-H1). COX3 and ND1 levels were expressed as copies/µl.
Renal COX3 and ND1 gradients were calculated by subtracting IVC from RV levels assess release of mtDNA specifically from the kidney.18 We have previously shown that the difference between infrarenal IVC and RV levels reflects the release of injury markers within the kidney.18–20
Statistical analysis
Statistical analysis was performed with JMP version 10.0 (SAS Institute, Cary, NC). Data distribution was assessed by the Shapiro–Wilk test. Gaussian distributed data was expressed as mean ± SD, and compared using analysis of variance with Tukey’s post-hoc, whereas nonnormally distributed data were expressed as medium (interquartile range 25–75%), and compared using Wilcoxon and Kruskal–Wallis nonparametric tests with Steel–Dawss post-hoc. Regressions were calculated by the least-squares fit. Urinary mtDNA levels were log-transformed. Univariate and multivariate models were applied to evaluate ethnicity, age, gender, body mass index, estimated GFR, and urinary protein effects on urinary COX3 and ND1. A P value <0.05 was considered statistically significant.
RESULTS
Table 1 shows the clinical, laboratory, and demographic characteristics of the study patients. Body mass index was higher, whereas age was lower and fewer women were recruited in ObAAEH compared with HV and CEH patients. Both systolic blood pressure and mean arterial pressure were similarly higher in CEH and ObAAEH compared to HV, whereas diastolic blood pressure did not differ among the groups. Duration of hypertension and number of antihypertensive drugs and statins were comparable between hypertensive groups. Total cholesterol, low-density lipoprotein, as well as serum and urine creatinine, urine protein, and protein/creatinine ratio were similar among the groups. However, estimated GFR was higher in ObAAEH compared to HV and CEH, and measured GFR higher in ObAAEH compared to CEH. Urinary sodium and FeNa were similar between hypertensive groups (P > 0.05), whereas filtered sodium and total sodium reabsorbed were higher in ObAAEH compared to CEH.
Table 1.
Clinical, laboratory, and demographic data of healthy volunteers (HV), Caucasian essential hypertensive (CEH), and Obese African American EH (ObAAEH) patients
| Parameter | HV | CEH | ObAAEH |
|---|---|---|---|
| Number of patients | 23 | 23 | 23 |
| Age (years) | 67 (55–76) | 67 (54–72) | 50 (47–53)*† |
| Gender (female/male) | 12/11 | 13/10 | 5/18*† |
| Body mass index | 26.4 (23.4–29.6) | 26.4 (23.9–30.6) | 31.6 (25.9–37.1)*† |
| Duration of hypertension (years) | – | 17.7 ± 4.0 | 8.9 ± 2.3 |
| No. of antihypertensive drugs (median) | 0 | 2.9 ± 1.3* | 2.4 ± 0.9* |
| Systolic blood pressure (mm Hg) | 115.9 ± 11.4 | 136.4 ± 18.7* | 134.0 ± 18.9* |
| Diastolic blood pressure (mm Hg) | 67.9 ± 9.4 | 71.7 ± 10.6 | 80.7 ± 13.5 |
| Mean blood pressure (mm Hg) | 83.9 ± 9.1 | 93.3 ± 10.7* | 98.4 ± 13.7* |
| Total cholesterol (mg/dl) | 167.8 ± 28.6 | 186.1 ± 30.7 | 190.8 ± 36.2 |
| Low-density lipoprotein (mg/dl) | 94.8 ± 29.7 | 106.1 ± 23.4 | 111.7 ± 33.8 |
| Statins (number/percentage) | 0 | 7 (30.4)* | 4 (17.39)* |
| Serum creatinine (mg/dl) | 1.0 (0.7–1.2) | 0.9 (0.5–1.6) | 0.8 (0.4–1.6) |
| eGFR-CDK-EPI (ml/min/1.73 m2) | 76.5 (66.4–85.0) | 85.6 (64.0–94.5) | 97.4 (82.6–114.1)*† |
| Iothalamate GFR (ml/min/1.73 m2) | – | 79.9 ± 21.1 | 95.9 ± 26.0† |
| Filtered sodium (mEq/day × 1,000) | – | 11159.9 ± 3077.9 | 14,675.0 ± 6,032.7† |
| Urinary sodium (mmol/24 hour) | – | 164.8 (106.1–277.7) | 173.6 (86.5–516.9) |
| Fractional excretion of sodium (%) | – | 2.9 (0.7–12.4) | 2.9 (0.9–2.7) |
| Total sodium reabsorbed (mEq/day) | – | 11128.9 ± 2974.2 | 13316.4 ± 3685.3† |
| Urinary protein (mg/dl)‡ | 63.0 (26.0–112.0) | 55.4 (37.8–111.2) | 48.0 (30.0–69.0) |
| Urinary creatinine (mg/dl) | 44.1 (9.6–175.1) | 36.4 (9.6–155.6) | 44.1 (20.6–65.5) |
| Urinary protein/creatinine ratio | 1.3 (0.5–3.7) | 1.3 (0.9–3.5) | 1.1 (0.7–1.5) |
Abbreviations: eGFR-CKD-EPI: estimated glomerular filtration rate-chronic kidney disease epidemiology collaboration.
*P < 0.05 vs. HV, †P < 0.05 vs. CEH; ‡Determined from 24 hour collection in CEH and ObAAEH.
Urinary and plasma mtDNA
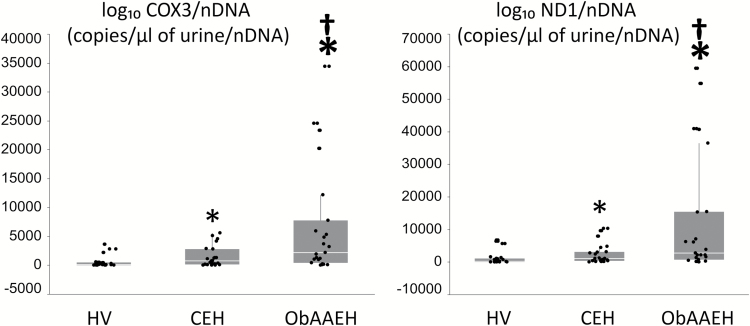
Urinary COX3 and ND1 were higher in CEH compared to HV, and further elevated in ObAAEH patients (Figure 1), whereas RNAse-P copy number did not differ among the groups (P = NS). Hence, the increase in COX3 and ND1 was specific to mtDNA rather than overall cellular DNA.
Figure 1.
Urinary mtDNA copy number is elevated in ObAAEH patients. Urinary copy number of cytochrome-c oxidase-3 (COX3) and NADH dehydrogenase subunit-1 (ND1) in HV, CEH, and ObAAEH patients. *P ≤ 0.05 vs. HV, †P ≤ 0.05 vs. CEH. Abbreviations: CEH, Caucasian essential hypertensive; HV, healthy volunteers; mtDNA, mitochondrial DNA; NADH, nicotinamide adenine dinucleotide; ObAAEH, Obese African American essential hypertensive.
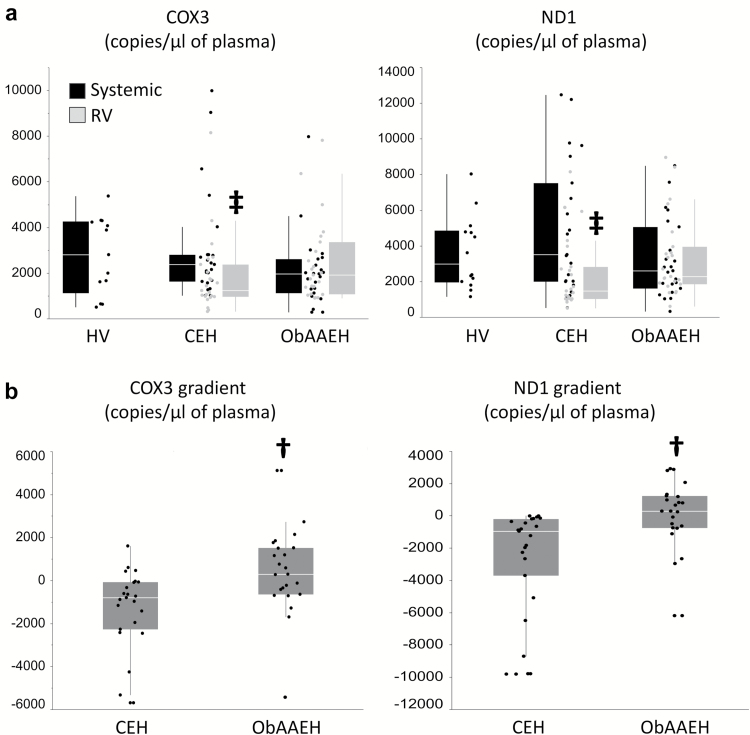
Systemic and RV plasma levels of COX3 and ND1 were similar among the groups, but only in CEH patients, levels of COX3 and ND1 were lower in the RV vs. IVC (Figure 2a). Consequently, the cross-kidney gradients of mtDNA were higher in ObAAEH compared to CEH (Figure 2b). Statistical differences in urinary COX3 and ND1 copy number between the groups persisted after correction for urinary creatinine (P < 0.0001 and P < 0.001, respectively).
Figure 2.
Plasma mtDNA developed a positive gradient across the kidney. (a): Neither systemic nor RV mtDNA levels differ between CEH and ObAAEH, but levels of COX3 and ND1 were lower in the RV vs. IVC of CEH patients. (b) COX3 and ND1 gradient across the kidney was higher in ObAAEH compared to CEH. †P ≤ 0.05 vs. CEH, ‡P ≤ 0.05 vs. systemic. Abbreviations: CEH, Caucasian essential hypertensive; HV, healthy volunteers; mtDNA, mitochondrial DNA; IVC, inferior vena cava; ObAAEH, Obese African American essential hypertensive; RV, renal vein.
Urinary mtDNA levels differed by ethnicity, age, gender, and GFR in univariate comparison, but in multivariate analysis, GFR remained the only predictor of elevated urinary COX3 and ND1 levels (Supplementary Table S1).
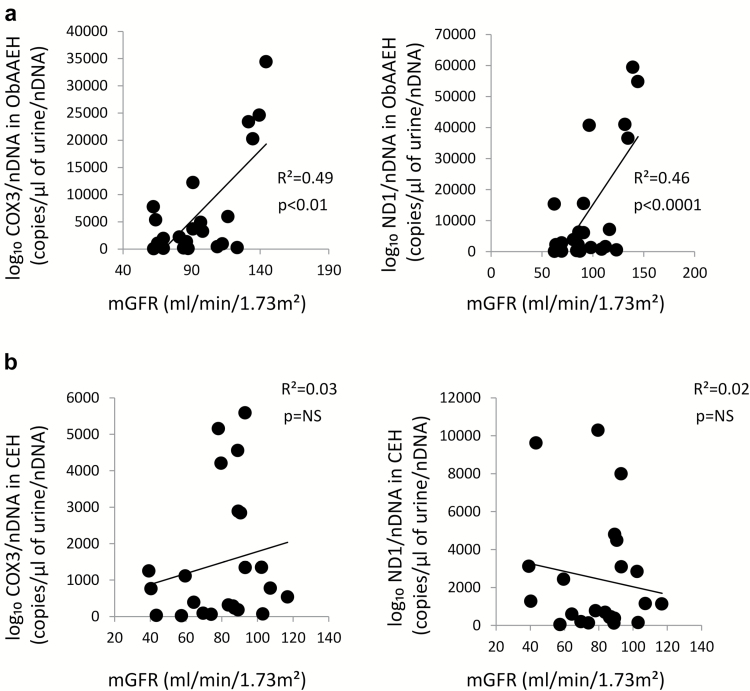
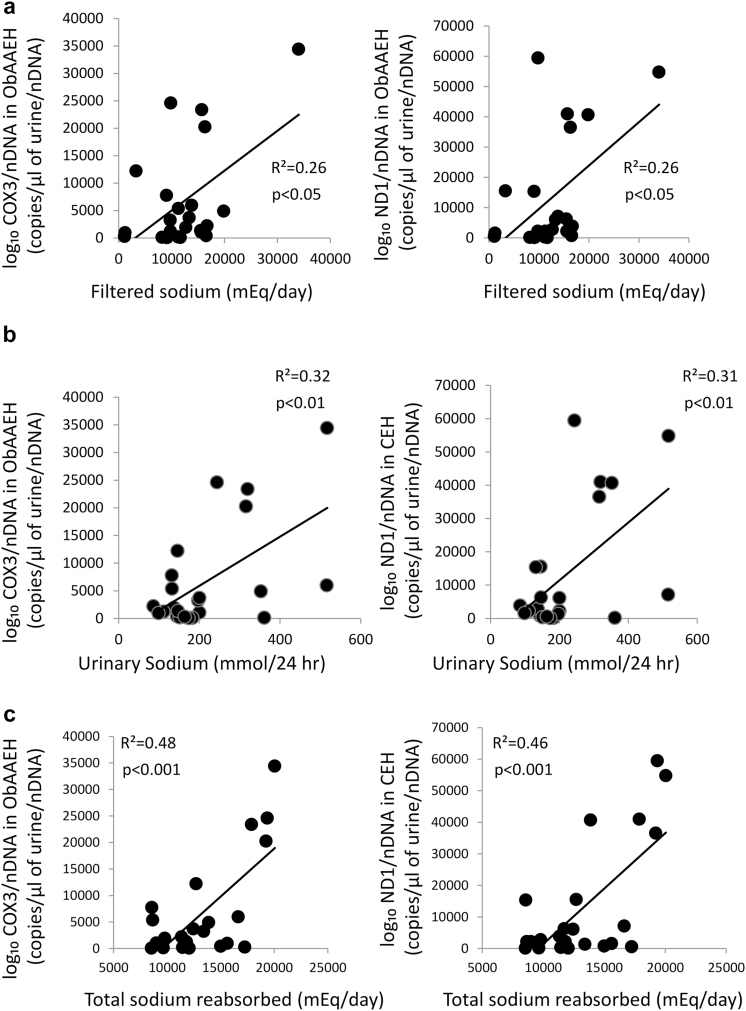
In ObAAEH (but not in CEH) patients, urinary COX3 and ND1 directly correlated with GFR (Figure 3a), whereas in CEH GFR did not correlate with either urinary COX3 or ND1 (Figure 3b). Urinary mtDNA copy number did not correlate with systolic blood pressure, diastolic blood pressure, or mean arterial pressure in either CEH or ObAAEH (all P = NS). Filtered sodium, urinary sodium, and particularly total sodium reabsorbed correlated directly with urinary mtDNA (Figure 4), but FeNa did not correlate with either urinary COX3 or ND1 (both P > 0.05).
Figure 3.
Urinary mtDNA copy number correlates with renal hyperfiltration. In ObAAEH patients, urinary COX3 and ND1 copy number correlated directly with mGFR (a), whereas in CEH mGFR did not correlate with either urinary COX3 or ND1 (b). Abbreviations: CEH, Caucasian essential hypertensive; mGFR, measured glomerular filtration rate; mtDNA, mitochondrial DNA; NS, nonsignificant; ObAAEH, Obese African American essential hypertensive.
Figure 4.
Urinary mtDNA copy number correlates with filtered sodium and total sodium reabsorbed. In ObAAEH patients, urinary COX3 and ND1 copy number correlated directly with filtered sodium (a), urinary sodium (b), and total sodium reabsorbed (c). Abbreviations: mtDNA, mitochondrial DNA; ObAAEH, Obese African American essential hypertensive.
DISCUSSION
This current study shows that urinary levels of fragments of the mitochondrial genome are elevated in ObAAEH patients and correlate with glomerular hyperfiltration after correction for covariates. Furthermore, in ObAAEH, mtDNA developed a positive gradient across the kidney, implying selective renal release in both the urine and venous blood. These observations suggest that kidneys of ObAAEH subjects may exhibit mitochondria injury, which in turn may potentially aggravate renal damage and accelerate hypertension-related morbidity/mortality rates.
Renal failure appears earlier and progresses faster in ObAAEH compared to CEH, but the mechanisms underlying this racial disparity remain to be elucidated.21 Genetic susceptibility (variants within the apolipoprotein L122 and MYH923 genes) and salt sensitivity24 have been proposed as important contributing factors to support the increased risk of hypertensive renal disease. Furthermore, we have previously shown that RV and circulating inflammatory markers are elevated in ObAAEH patients and correlate with decreased endothelial progenitor cell levels, suggesting that impaired renal reparative capacity may predispose to hypertensive vascular injury.25,26 Yet, the contribution of mitochondrial injury to this disparity remained unclear.
Obesity is an important contributor to hypertension in all ethnic groups that causes structural changes in the kidneys and loss of nephron function.27 Glomerular hyperfiltration is an important obesity-related physiological effect commonly observed in ObAAEH patients.28 Importantly, this observation resulted in the inclusion of ethnicity as a component of the Modification of Diet in Renal Disease GFR equation.29 In patients with CEH, high creatinine clearance (suggesting glomerular hyperfiltration) is an important predictor for the development of early hypertensive nephropathy.30 The current model of “sodium glomerulopathy” also suggests that sodium sensitivity in ObAAEH results from an increased activity of Na-K-2Cl cotransport in the thick ascending limb of Henle’s loop,31 but the mechanisms by which glomerular hyperfiltration induces kidney damage in ObAAEH remain to be determined. The current study highlights a novel potential mechanism by which glomerular hyperfiltration may injure the kidney in ObAAEH. We found that urinary COX3 and ND1 levels were elevated in ObAAEH patients and directly correlated with GFR, suggesting that mitochondrial injury may result from and/or aggravate hypertension-related renal damage in ObAAEH.
Previous studies have shown that urinary mtDNA, which may escape from injured cellular mitochondria to the urine or systemic circulation, is a biomarker of mitochondrial disruption and renal dysfunction in acute kidney injury.12 In agreement, we have previously shown that urinary mtDNA is elevated in hypertensive patients and correlates with markers of renal injury and dysfunction, suggesting that mitochondria are implicated in renal injury and might represent a novel therapeutic target in hypertension.14 A previous study has reported mitochondrially encoded gene mutations associated with renal dysfunction in peripheral blood lymphocytes of ObAAEH, suggesting that mtDNA may account for a subgroup of ObAAEH with end-stage renal disease.32 In order to cover the entire spectrum of the mitochondrial respiratory chain, we measured urinary levels of COX3 and ND1, which encode for subunits of the terminal enzyme of the mitochondrial respiratory chain (complex-III) and the enzyme responsible for the first step in the electron transport (complex-I), respectively. Furthermore, these genes are located in opposite regions of the circular mtDNA.33 Hence, we ensured adequate functional and anatomical representation of mtDNA. Previous studies have shown that urinary levels of COX3 and ND1 were similarly elevated in patients with AKI, reflecting renal mitochondrial damage.12,13 In this study, we found higher urinary mtDNA levels in ObAAEH that correlated directly with GFR, extending previous observations and implicating mitochondrial injury in the consequences of glomerular hyperfiltration in ObAAEH subjects.
Furthermore, we provide evidence demonstrating renal release of mtDNA into the systemic circulation, reflected in a positive gradient across the kidney. We have previously shown that the cross-kidney gradients of inflammatory markers reflect their release within the affected kidney.18 Importantly, mtDNA has potent proinflammatory properties via Toll-Like Receptor (TLR)-9 mediated responses, which may induce inflammation in remote organs.34 Indeed, circulating mtDNA levels are elevated in maintenance hemodialysis patients, and closely correlate with chronic inflammation.35 However, the unchanged circulating levels of COX3 and ND1 among the groups are inconsistent with mtDNA-induced systemic inflammation in our study.
The mechanisms linking mitochondrial injury and glomerular hyperfiltration remain unclear, and are likely multifactorial. Obesity and hypertension, which are commonly associated with mechanical stretch, increased production of reactive oxygen species, extracellular matrix turnover, and fibrosis, may alter the structure and function of the mitochondrion.11 Furthermore, in the current study, glomerular hyperfiltration in ObAAEH was associated with increased filtered sodium, but urinary sodium levels and FeNa were similar between hypertensive groups. Interestingly, total sodium reabsorbed was higher in ObAAEH compared to CEH, and correlated better with urinary COX3 and ND1 levels than filtered sodium, suggesting that increased sodium reabsorption may contribute to renal mitochondrial injury in ObAAEH. Further experimental and clinical research is needed to explore the precise cause and effect relationships between mitochondrial injury and hyperfiltration in ObAAEH patients.
Speculatively, changes in GFR might be partly due to mitochondrial damage of the cellular components of the glomerular filtration membrane. This highly specialized structure ensures selective ultrafiltration of plasma and is primarily composed by podocytes and endothelial cells.36 Furthermore, increased glomerular pressure and stretch may lead to cellular injury and mitochondrial fragmentation. Substantial levels of energy are required to maintain podocyte structure and function, which is primarily derived from the respiratory chain of the inner mitochondrial membrane.37 Therefore, renal mitochondrial injury may result in podocyte damage and loss of barrier function. Likewise, glomerular endothelial cell mitochondrial injury may compromise the integrity of the filtration barrier.38 Further studies are needed to explore the mechanisms and consequences of hypertension-induced renal mitochondrial injury.
Because of the prospective and well-controlled nature of our study, as well as RV catheterization, our study is limited by its cross-sectional nature and the relatively small number of participants. Patients were not specifically matched by age and gender; age was lower and fewer women were recruited in ObAAEH compared with HV and CEH patients. Nevertheless, none of these parameters remained predictors of elevated urinary mtDNA levels in multivariate analysis. Incidentally, a wide range of GFR values resulted in a spectrum of mtDNA values in our patients. Despite significant correlations between GFR and urinary mtDNA levels, we cannot rule out the possibility that other factors may have contributed to mitochondrial damage in ObAAEH. For example, angiotensin-II receptor activation in the inner mitochondrial membrane may induce mitochondrial dysfunction,39 but blockade of the renin–angiotensin–aldosterone system in the hypertensive groups argue against this mechanism. Whether treatment with antihypertensive drugs may alter urinary COX3 and ND1 levels requires future studies. We have not measured urinary levels of enzymes, which could degrade DNA, but samples were frozen immediately to avoid catalytic degradation.
In summary, this study shows that ObAAEH subjects have increased levels of markers of mitochondrial injury, as measured by urinary COX3 and ND1 copy number. Furthermore, urinary mtDNA levels correlate directly with GFR, suggesting that mitochondria may be implicated in glomerular hyperfiltration. These observations may position mitochondria as novel therapeutic targets to attenuate hypertension-related morbidity and mortality rates in ObAAEH.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension online.
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENT
This study was partly supported by the NIH grant numbers: DK100081, DK73608, DK104273, HL123160, DK102325, and DK106427. This manuscript was sent to Guest Editor, Charles T. Stier, Jr., PhD for editorial handling and final disposition.”
REFERENCES
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; Writing Group Members; American Heart Association Statistics Committee; Stroke Statistics Subcommittee Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016; 133:e38–360. [DOI] [PubMed] [Google Scholar]
- 2. Ortega LM, Sedki E, Nayer A. Hypertension in the African American population: a succinct look at its epidemiology, pathogenesis, and therapy. Nefrologia 2015; 35:139–145. [DOI] [PubMed] [Google Scholar]
- 3. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298:2038–2047. [DOI] [PubMed] [Google Scholar]
- 4. Kotchen TA, Piering AW, Cowley AW, Grim CE, Gaudet D, Hamet P, Kaldunski ML, Kotchen JM, Roman RJ. Glomerular hyperfiltration in hypertensive African Americans. Hypertension 2000; 35:822–826. [DOI] [PubMed] [Google Scholar]
- 5. Parmer RJ, Stone RA, Cervenka JH. Renal hemodynamics in essential hypertension. Racial differences in response to changes in dietary sodium. Hypertension 1994; 24:752–757. [DOI] [PubMed] [Google Scholar]
- 6. Textor SC, Gloviczki ML, Flessner MF, Calhoun DA, Glockner J, Grande JP, McKusick MA, Cha SS, Lerman LO. Association of filtered sodium load with medullary volumes and medullary hypoxia in hypertensive African Americans as compared with Whites. Am J Kidney Dis 2012; 59:229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hall AM, Unwin RJ. The not so “mighty chondrion”: emergence of renal diseases due to mitochondrial dysfunction. Nephron Physiol 2007; 105:p1–10. [DOI] [PubMed] [Google Scholar]
- 8. McFarland R, Taylor RW, Turnbull DM. Mitochondrial disease–its impact, etiology, and pathology. Curr Top Dev Biol 2007; 77:113–155. [DOI] [PubMed] [Google Scholar]
- 9. Hajnóczky G, Csordás G, Das S, Garcia-Perez C, Saotome M, Sinha Roy S, Yi M. Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium 2006; 40:553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murphy E, Ardehali H, Balaban RS, DiLisa F, Dorn GW, 2nd, Kitsis RN, Otsu K, Ping P, Rizzuto R, Sack MN, Wallace D, Youle RJ; American Heart Association Council on Basic Cardiovascular Sciences, Council on Clinical Cardiology, and Council on Functional Genomics and Translational Biology Mitochondrial function, biology, and role in disease: a scientific statement from the American Heart Association. Circ Res 2016; 118:1960–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eirin A, Lerman A, Lerman LO. Mitochondria: a pathogenic paradigm in hypertensive renal disease. Hypertension 2015; 65:264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Whitaker RM, Stallons LJ, Kneff JE, Alge JL, Harmon JL, Rahn JJ, Arthur JM, Beeson CC, Chan SL, Schnellmann RG. Urinary mitochondrial DNA is a biomarker of mitochondrial disruption and renal dysfunction in acute kidney injury. Kidney Int 2015; 88:1336–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hu Q, Ren J, Wu J, Li G, Wu X, Liu S, Wang G, Gu G, Ren H, Hong Z, Li J. Urinary mitochondrial DNA levels identify acute kidney injury in surgical critical illness patients. Shock 2017. [DOI] [PubMed] [Google Scholar]
- 14. Eirin A, Saad A, Tang H, Herrmann SM, Woollard JR, Lerman A, Textor SC, Lerman LO. Urinary mitochondrial DNA copy number identifies chronic renal injury in hypertensive patients. Hypertension 2016; 68:401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kho AN, Pacheco JA, Peissig PL, Rasmussen L, Newton KM, Weston N, Crane PK, Pathak J, Chute CG, Bielinski SJ, Kullo IJ, Li R, Manolio TA, Chisholm RL, Denny JC. Electronic medical records for genetic research: results of the eMERGE consortium. Sci Transl Med 2011; 3:79re1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wilson DM, Bergert JH, Larson TS, Liedtke RR. GFR determined by nonradiolabeled iothalamate using capillary electrophoresis. Am J Kidney Dis 1997; 30:646–652. [DOI] [PubMed] [Google Scholar]
- 18. Eirin A, Gloviczki ML, Tang H, Gössl M, Jordan KL, Woollard JR, Lerman A, Grande JP, Textor SC, Lerman LO. Inflammatory and injury signals released from the post-stenotic human kidney. Eur Heart J 2013; 34:540–548a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eirin A, Gloviczki ML, Tang H, Rule AD, Woollard JR, Lerman A, Textor SC, Lerman LO. Chronic renovascular hypertension is associated with elevated levels of neutrophil gelatinase-associated lipocalin. Nephrol Dial Transplant 2012; 27:4153–4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eirin A, Zhang X, Zhu XY, Tang H, Jordan KL, Grande JP, Dietz AB, Lerman A, Textor SC, Lerman LO. Renal vein cytokine release as an index of renal parenchymal inflammation in chronic experimental renal artery stenosis. Nephrol Dial Transplant 2014; 29:274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Judd E, Calhoun DA. Obesity, African American race, chronic kidney disease, and resistant hypertension: the step beyond observed risk. Hypertension 2016; 67:275–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 2010; 329:841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kopp JB, Smith MW, Nelson GW, Johnson RC, Freedman BI, Bowden DW, Oleksyk T, McKenzie LM, Kajiyama H, Ahuja TS, Berns JS, Briggs W, Cho ME, Dart RA, Kimmel PL, Korbet SM, Michel DM, Mokrzycki MH, Schelling JR, Simon E, Trachtman H, Vlahov D, Winkler CA. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet 2008; 40:1175–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weir MR, Fink JC. Salt intake and progression of chronic kidney disease: an overlooked modifiable exposure? A commentary. Am J Kidney Dis 2005; 45:176–188. [DOI] [PubMed] [Google Scholar]
- 25. Eirin A, Zhu XY, Woollard JR, Herrmann SM, Gloviczki ML, Saad A, Juncos LA, Calhoun DA, Rule AD, Lerman A, Textor SC, Lerman LO. Increased circulating inflammatory endothelial cells in Blacks with essential hypertension. Hypertension 2013; 62:585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim SR, Eirin A, Herrmann SM, Saad A, Juncos LA, Lerman A, Textor SC, Lerman LO. Preserved endothelial progenitor cell angiogenic activity in African American essential hypertensive patients. Nephrol Dial Transplant 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hall JE, Kuo JJ, da Silva AA, de Paula RB, Liu J, Tallam L. Obesity-associated hypertension and kidney disease. Curr Opin Nephrol Hypertens 2003; 12:195–200. [DOI] [PubMed] [Google Scholar]
- 28. Wuerzner G, Pruijm M, Maillard M, Bovet P, Renaud C, Burnier M, Bochud M. Marked association between obesity and glomerular hyperfiltration: a cross-sectional study in an African population. Am J Kidney Dis 2010; 56:303–312. [DOI] [PubMed] [Google Scholar]
- 29. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130:461–470. [DOI] [PubMed] [Google Scholar]
- 30. Schmieder RE, Veelken R, Gatzka CD, Rüddel H, Schächinger H. Predictors for hypertensive nephropathy: results of a 6-year follow-up study in essential hypertension. J Hypertens 1995; 13:357–365. [PubMed] [Google Scholar]
- 31. Aviv A, Hollenberg NK, Weder AB. Sodium glomerulopathy: tubuloglomerular feedback and renal injury in African Americans. Kidney Int 2004; 65:361–368. [DOI] [PubMed] [Google Scholar]
- 32. Watson B, Jr, Khan MA, Desmond RA, Bergman S. Mitochondrial DNA mutations in Black Americans with hypertension-associated end-stage renal disease. Am J Kidney Dis 2001; 38:529–536. [DOI] [PubMed] [Google Scholar]
- 33. Bogenhagen DF. Mitochondrial DNA nucleoid structure. Biochim Biophys Acta 2012; 1819:914–920. [DOI] [PubMed] [Google Scholar]
- 34. Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, Akira S, Yamamoto A, Komuro I, Otsu K. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 2012; 485:251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cao H, Ye H, Sun Z, Shen X, Song Z, Wu X, He W, Dai C, Yang J. Circulatory mitochondrial DNA is a pro-inflammatory agent in maintenance hemodialysis patients. PLoS One 2014; 9:e113179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dimke H, Maezawa Y, Quaggin SE. Crosstalk in glomerular injury and repair. Curr Opin Nephrol Hypertens 2015; 24:231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Müller-Deile J, Schiffer M. The podocyte power-plant disaster and its contribution to glomerulopathy. Front Endocrinol (Lausanne) 2014; 5:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tanaka T, Miyata T, Inagi R, Kurokawa K, Adler S, Fujita T, Nangaku M. Hypoxia-induced apoptosis in cultured glomerular endothelial cells: involvement of mitochondrial pathways. Kidney Int 2003; 64:2020–2032. [DOI] [PubMed] [Google Scholar]
- 39. Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 2008; 102:488–496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.