Abstract
BACKGROUND
The association of electrocardiographic left ventricular hypertrophy (ECG-LVH) with blood pressure (BP) in Blacks living in sub-Saharan Africa remains poorly documented.
METHODS
In 225 Black Nigerians and 729 White Flemish, we analyzed QRS voltages and voltage-duration products and 12 criteria diagnostic of ECG-LVH in relation to office BP (mean of 5 consecutive readings) and home BP (duplicate morning and evening readings averaged over 1 week).
RESULTS
In multivariable analyses, QRS voltage and voltage-duration indexes were generally higher in Blacks than Whites. By using any of 12 criteria, ECG-LVH was more prevalent among Black than White men (54.4% vs. 36.0%) with no ethnic difference among women (17.1%). Precordial voltages and voltage-duration products increased with office and home systolic BP (SBP), and increases were up to 3-fold steeper in Blacks. In Blacks vs. Whites, increases in the Sokolow–Lyon voltage associated with a 10-mm Hg higher SBP were 0.18 mV (95% confidence interval [CI], 0.09–0.26) vs. 0.06 mV (0.02–0.09) and 0.17 mV (0.07–0.28) vs. 0.11 mV (CI, 0.07–0.15) for office and home BP, respectively, with a significant ethnic gradient (P < 0.05). The risk of ECG-LVH increased more with office and home BP in Blacks than Whites.
CONCLUSIONS
Associations of ECG voltages and voltage-duration products and risk of ECG-LVH with BP are steeper in Black Nigerians compared with a White reference population. In resource-poor settings of sub-Saharan Africa, the ECG in combination with office and home BP is an essential instrument in risk stratification across the entire BP range.
Keywords: blood pressure, electrocardiography, ethnicity, home blood pressure, hypertension, left ventricular hypertrophy, population science, risk stratification, special populations
Hypertension is a major cardiovascular risk factor with long-term effect on left ventricular structure and function.1 Left ventricular hypertrophy (LVH) diagnosed either by electrocardiography2,3 or imaging modalities4,5 is an independent predictor of cardiovascular events and mortality over and beyond other established risk factors. Left ventricular mass increases with blood pressure (BP) in both Blacks and Whites, but more steeply in Blacks than in Whites.6–9 However, the Blacks included in previous studies were Americans of African ancestry.6–9 None of these studies6–9 assessed the association of electrocardiographic traits with the self-measured home BP. In addition, most of the previous studies assessed LVH using echocardiography or electrocardiography calibrated against echocardiography.7 Although echocardiography7 and other cardiac imaging modalities10 give a more accurate estimate of the anatomical left ventricular mass than electrocardiography, the latter technique provides unique information on electrical properties of the pathologically changed myocardium.5,11 In addition, an electrocardiogram is easy to acquire and is cost effective when compared to imaging approaches.7,10 As such it is an attractive method of assessing LVH that will ensure wide clinical applicability of research findings in resource-poor settings characteristic of many regions in sub-Saharan Africa. Therefore, we assessed ethnic differences in the associations of QRS voltages and voltage-duration products and of electrocardiographic LVH as diagnosed by published criteria3,12–19 with office and home BPs in a group of Blacks Nigerians and a reference population-based cohort of White Flemish.
METHODS
Study population
The Nigerian and Flemish population studies complied with the Helsinki Declaration for investigation of human subjects.20 They received ethical approval from the University of Abuja Teaching Hospital Health Research Ethics Committee and the Faculty of Medicine of the University of Leuven. All participants provided informed written consent.
Nigerian population study.
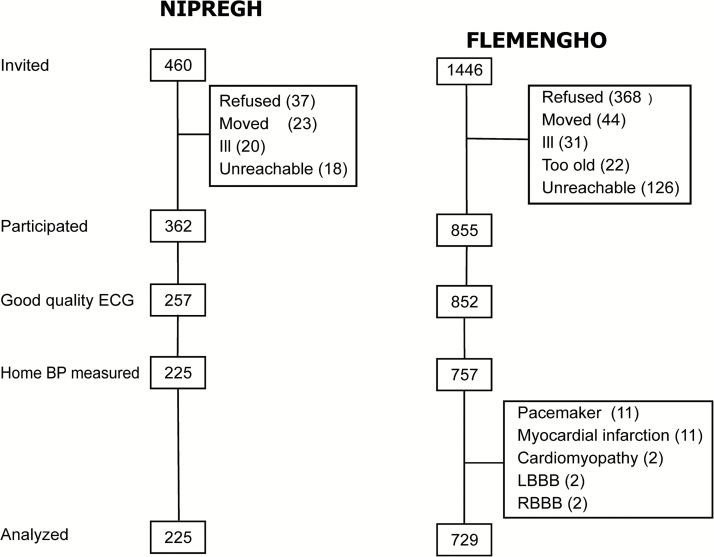
The Black Nigerian population consisted of participants recruited in the framework of the ongoing Nigerian Population Research on Environment, Gene and Health (NIPREGH)21 which commenced in April 2013. Eligible adults were living in a well-delineated housing estate in Abuja. They were invited to a local examination center for a physical examination and electrocardiography. The participation rate was 79%. A high-quality electrocardiogram could not be obtained in 137 of the 362 participants because of frequent and unpredictable power failures in the catchment area and the poor grounding of the electrical wiring in the examination center. We excluded another 32 individuals because less than 2 home BP measurements were available. Thus, the number of Nigerians analyzed totaled 225. They had no history of heart disease (Figure 1).
Figure 1.
Flow chart of Black and White participants enrolled in the Nigerian Population Research on Environment Gene and Health (NIPREGH21) and the Flemish Study on Environment, Genes and Health Outcomes (FLEMENGHO22). Abbreviations: BP, blood pressure; ECG, electrocardiogram; LBBB, left bundle branch block; RBBB, right bundle branch block.
Flemish Population Study.
The White Flemish had been enrolled in the Flemish Study on Environment, Genes and Health Outcomes (FLEMENGHO).22 Recruitment started in 1985 and continued until 2004. The initial participation rate was 78%. The participants were repeatedly followed-up. Self-measurement of BP at home started in September 2012. Since then, 1,446 former participants were invited for a follow-up examination at a local examination center. However, 97 were unavailable, because they had been institutionalized or were too ill (n = 53), or had moved out-of-the area or did not respond (n = 44). Of the remaining 1,349 former participants, 855 renewed informed consent (63%). We excluded 126 participants from analysis, because the electrocardiogram was either missing or of insufficient quality (n = 3), because less than 2 self-recorded BP measurements were available (n = 95), because they were pacemaker-dependent (n = 11), had a history of myocardial infarction (n = 11), cardiomyopathy (n = 2), or because their electrocardiogram showed left or right bundle branch block (n = 4). Thus, the number of White participants statistically analyzed totaled 729 (Figure 1).
Electrocardiography
Standard 12-lead electrocardiograms were recorded at a speed of 25 mm/sec with the calibration set at 1 mV/cm. To improve the quality of the electrocardiograms, research assistants received periodical training on skin preparation, electrode placement and positioning of the subjects. Certified cardiologists checked the electrocardiograms. The Cardiax device (RDSM Medical Devices, Hasselt, Belgium) was used in NIPREGH21 and the Schiller-AT2 device (Schiller Medizintechnik GmbH, Feldkirchen bei München, Germany) in FLEMENGHO.22 These devices are equipped with software that automatically measures the amplitudes and the duration of the waves in each of the 12 leads. All measurements were exported into Excel and imported in SAS. Low-frequency noise emanating from movement, baseline wander, and respiration and high frequency noise emanating from power-line or radiated electromagnetic influence were filtered before the final signal acquisition. In accordance with the recommendations of the American Heart Association,23 cutoff values were set at 0.05 Hz and 150 Hz for the low- and high-frequency filters, respectively. LVH was diagnosed using 12 published criteria (Table 1), based on voltages of the limb3,12 or precordial12–14 leads or their combination23,24 or on QRS voltage × duration products.18,19 These criteria were calculated from the exported voltages and durations using a standardized SAS program. Outliers were checked against the source data and the printed electrocardiograms.
Table 1.
Electrocardiographic criteria for left ventricular hypertrophy
| Criterion (reference) | Definition | LVH threshold |
| Limb-lead voltages | ||
| Lewis3 | (R in I − S in I) + (S in III − R in III) | >1.6 mV |
| Gubner–Ungerleider3 | R in I + S in III | >2.5 mV |
| Sokolow–Lyon12 | R in aVL | >1.1 mV |
| Precordial lead voltages | ||
| Sokolow-Lyon12 | S in V1 + maximum of R in V5, and R in V6 | >3.5 mV |
| Wilson13 | R in V5 | >3.3 mV |
| Romhilt14 | S in V2 + maximum of R in V5 or V6 | >4.5 mV |
| Combination of limb and precordial voltages | ||
| Cornell in women15 | S in V3 + R in aVL | >2.0 mV |
| Cornell in men15 | S in V3 + R in aVL | >2.8 mV |
| Siegel total voltage16 | Sum of greatest positive and negative QRS deflection in 12 leads | >17.5 mV |
| Manning ≤30 years17 | Sum of Q, R and S voltages in aVF, V2, and V6 | >5.9 mV |
| Manning >30 years17 | Sum of Q, R and S voltages in aVF, V2, and V6 | >9.3 mV |
| Voltage × QRS duration products | ||
| Sokolow–Lyon18 | Sokolow–Lyon index × QRS duration | >288.0 mV.ms |
| Cornell (women)19 | (Cornell index + 0.8 mV) × QRS duration | >243.6 mV.ms |
| Cornell (men)19 | Cornell index × QRS duration | >243.6 mV.ms |
| Siegel19 | (Siegel total voltage) × QRS duration | >1747.2 mV.ms |
Abbreviation: LVH, left ventricular hypertrophy.
BP measurement
NIPREGH21 and FLEMENGHO22 applied similar protocols for both office and home BP measurement. Trained observers measured office BP by auscultation of the Korotkoff sounds at the nondominant arm, according to the guidelines of the European Society of Hypertension.25 After the participants had rested in the sitting position for at least 10 minutes, the observers obtained 5 BP readings at an interval of 30 to 60 seconds. Systolic and phase V diastolic BPs were determined to the nearest 2 mm Hg. Standard cuffs had a 12 × 24 cm inflatable portion, but if upper arm girth exceeded 32 cm, larger cuffs with 15 × 35 cm bladders were used. All study participants were trained on how to measure BP at home, using a validated semi-automated oscillometric device (Omron 705 IT26 in NIPREGH and Omron M10 IT in FLEMENGHO27; Hoofddorp, The Netherlands). To demonstrate compliance with the technique of self-measurement, participants were invited to take 2 consecutive measurements immediately after the instructions for BP self-measurement had been orally explained. Next, the monitor was handed over to the participant for recording the self-measured BP at home. In line with European guidelines,25 participants were asked to record their BP on 7 consecutive days, 2 readings in the morning between 6 am and 8 am before breakfast and intake of medications and twice in the evening between 7 pm and 9 pm before going to sleep. At the end of the recording period, all BP readings were exported by the Omron software and imported into SAS. The home BP was the average of all self-measured readings.
Other measurements
The observers measured each participants’ anthropometric characteristics and administered a standard questionnaire to collect information about their medical history, smoking and drinking habits, and intake of medications. Skinfold thickness was the average of measurements obtained at 3 sites, i.e. the triceps, subscapular area, and suprailiac crest, by means of the Harpenden Skinfold Caliper (Bedfordshire, UK) providing a constant pressure of 0.01 kg per mm2 (0.098 N/mm2) at all openings of the 90 mm2 anvils. Body mass index was body weight in kilograms divided by height in meters squared. Diabetes mellitus was the use of antidiabetic drugs or a fasting or random plasma glucose equal to or exceeding 7.0 or 11.1 mmol/l.28
Statistical analysis
For database management and statistical analysis, we used SAS software version 9.4. (SAS Institute, Cary, NC). We reported the central tendency and spread of the data as mean and SD. For comparison of means and proportions, we applied the large sample z-test and the χ2 statistic, respectively. Statistical significance was a P value less than 0.05 on 2-sided tests. We standardized the prevalence of LVH by the direct method for sex and age (<45 vs. ≥45 years). We used multiple linear regression to adjust for confounders, while assessing the relation of the electrocardiographic variables as continuous variables with the office and home BPs. Using multiple logistic regression models, we determined the odds ratio of having LVH associated with a 10 mm Hg higher office or home systolic BP (SBP). We tested heterogeneity among Blacks and Whites by introducing the appropriate interaction terms in the model.
RESULTS
Characteristics of participants
Table 2 lists the characteristics of the participants by sex and ethnicity. Compared to Whites, Blacks were younger (39.1 vs. 52.8 years; P < 0.0001) and had lower (P < 0.05) conventional (112/72 vs. 132/83 mm Hg) and home (116/75 vs. 122/76 mm Hg) SBP and diastolic BP. Compared to White men, Black men had lower (P < 0.0001) body mass index (25.1 vs. 27.4 kg/m2), waist-to-hip ratio (0.91 vs. 0.94), and skinfold thickness (1.4 vs. 2.1 mm). On the other hand, Black women had a higher waist-to-hip ratio (0.88 vs. 0.86; P = 0.048) but thinner skinfolds (1.9 vs. 2.6 mm) compared with White women. The prevalence of smoking (2% vs. 13%) and drinking alcohol (30% vs. 70%) and using BP lowering drugs was lower (P < 0.01) among Blacks than Whites.
Table 2.
Characteristics of participants by sex and ethnicity
| Characteristics | Women | Men | ||
| Blacks | Whites | Blacks | Whites | |
| Number in category | 111 | 376 | 114 | 353 |
| Number with characteristic (%) | ||||
| Smoking | 0 (0.0) | 46 (12.2)§ | 4 (3.5) | ≤49 (13.9)† |
| Drinking alcohol | 25 (22.5) | 219 (58.2)§ | 43 (37.7) | 290 (82.2)§ |
| Office hypertension | 22 (19.8) | 156 (41.5)§ | 22 (19.3) | 197 (55.8)§ |
| Home hypertension | 28 (25.2) | 132 (35.1) | 28 (24.6) | 157 (44.5)‡ |
| Antihypertensive medication | 20 (18.0) | 91 (24.2) | 16 (14.0) | 101 (28.6)† |
| Diabetes mellitus | 9 (8.1) | 14 (3.7) | 6 (5.3) | 19 (5.4) |
| Body mass index >30 kg/m² | 32 (28.8) | 79 (21.0) | 15 (13.2) | 83 (23.5)* |
| Mean characteristic ± SD | ||||
| Age, years | 38.3 (11.3) | 52.7 (15.8)§ | 39.8 (10.1) | 53.0 (15.0)§ |
| Body weight, kg | 71.6 (15.3) | 70.3 (14.5) | 76.0 (13.2) | 85.5 (14.0)§ |
| Body height, cm | 163.6 (5.7) | 163.6 (6.8) | 173.7 (7.4) | 176.6 (7.6)‡ |
| Body mass index, kg/m2 | 26.8 (5.5) | 26.2 (5.0) | 25.1 (3.6) | 27.4 (4.0)§ |
| Waist circumference, cm | 91.0 (12.6) | 89.9 (13.3) | 89.9 (12.4) | 97.8 (11.1)§ |
| Hip circumference, cm | 103.5 (12.5) | 103.9 (10.3) | 98.8 (10.0) | 103.9 (6.9)§ |
| Waist-to-hip ratio | 0.88 (0.1) | 0.86 (0.07)* | 0.91 (0.06) | 0.94 (0.07)§ |
| Skinfold thickness, mm | 1.9 (0.9) | 2.6 (1.0)§ | 1.4 (0.8) | 2.1 (0.9)§ |
| Office systolic pressure, mm Hg | 108.8 (14.8) | 130.6 (18.2)§ | 114.9 (15.4) | 134.3 (15.2)§ |
| Office diastolic pressure, mm Hg | 68.8 (10.3) | 80.8 (9.4)§ | 74.7 (12.7) | 86.1 (9.4)§ |
| Home systolic pressure, mm Hg | 110.3 (14.6) | 118.2 (16.5)§ | 122.1 (12.6) | 124.9 (11.9)* |
| Home diastolic pressure, mm Hg | 72.1 (9.7) | 74.9 (8.4)† | 77.6 (10.1) | 78.1 (7.8) |
| Office heart rate, bpm | 74.0 (10.0) | 64.7 (8.7)§ | 70.8 (9.9) | 62.1 (8.9)§ |
Skinfold thickness was the average of measurements obtained at the triceps, subscapular area, and suprailiac crest. Office and home blood pressures were averages of 5 consecutive auscultatory readings or all home readings over 1 week. Office hypertension was a blood pressure ≥140 mm Hg systolic or ≥90 mm Hg diastolic. The corresponding thresholds for the home blood pressure were ≥135 mm Hg and ≥85 mm Hg. Patients on blood pressure lowering drugs were classified as hypertensive. Diabetes is a fasting or random blood glucose >7.0 or >11.1 mmol/l, respectively, or use of antidiabetic drugs. Significance of the between-cohort differences: *P < 0.05; †P < 0.01; ‡P < 0.001; and §P < 0.0001.
Electrocardiographic measurements according to ethnicity
We assessed LVH according to 12 different criteria (Table 1), unadjusted and with adjustment for age, body mass index, skinfold thickness, and antihypertensive drug intake. Supplementary Table S1 (unadjusted) and Table 3 (adjusted) show the distribution of these measurements on a continuous scale broken down by sex and ethnicity. With adjustments applied, the limb-lead voltages (P < 0.001) and the Sokolow–Lyon index (P < 0.05) were higher in Black women and men compared with their White counterparts. On the contrary, the Siegel and Manning criteria based on a combination of limb and precordial voltages were lower (P < 0.05) in Black Nigerians compared to White Flemish. The ethnic differences in the voltage × duration products paralleled those in the corresponding voltage-only criteria.
Table 3.
Adjusted ECG measurements by sex and ethnicity
| ECG criteria | Women | Men | ||
| Blacks | Whites | Blacks | Whites | |
| Limb-lead voltages, mV | ||||
| Lewis3 | 0.73 (0.60) | −0.01 (0.73)§ | 0.83 (0.72) | 0.03 (0.78)§ |
| Gubner–Ungerleider3 | 0.92 (0.41) | 0.76 (0.39)‡ | 1.04 (0.53) | 0.81 (0.43)§ |
| Sokolow–Lyon12 | 0.39 (0.23) | 0.26 (0.22)‡ | 0.47 (0.29) | 0.28 (0.25)§ |
| Precordial lead voltages, mV | ||||
| Sokolow–Lyon12 | 2.20 (0.76) | 2.03 (0.63)* | 2.83 (1.00) | 2.37 (0.72)§ |
| Wilson13 | 1.24 (0.44) | 1.25 (0.41) | 1.66 (0.73) | 1.61 (0.51) |
| Romhilt14 | 2.07 (0.79) | 2.19 (0.65) | 3.03 (1.13) | 2.67 (0.72)* |
| Combination of limb and precordial voltages, mV | ||||
| Cornell15 | 0.94 (0.46) | 1.00 (0.45) | 1.42 (0.72) | 1.21 (0.57)† |
| Siegel16 | 11.2 (3.57) | 13.4 (3.11)§ | 14.5 (4.18) | 15.6 (3.23)* |
| Manning17 | 2.80 (1.15) | 3.68 (1.06)§ | 3.80 (1.33) | 4.18 (1.07)† |
| Voltage × QRS duration products, mV.ms | ||||
| Sokolow–Lyon18 | 200.5 (70.5) | 175.9 (56.5)† | 264.3 (94.7) | 227.5 (71.9)‡ |
| Cornell19 | 160.6 (47.6) | 157.6 (47.1) | 134.5 (71.8) | 117.1 (59.1)* |
| Siegel19 | 1023.0 (353.4) | 1172.0 (335.6)§ | 1368.4 (433.4) | 1511.6 (389.6)† |
Values are mean (SD) adjusted for age, body mass index, skinfold thickness, and antihypertensive drug intake. Significance of the between-cohort differences: *P < 0.05; †P < 0.01; ‡P < 0.001; and §P < 0.0001.
The significant differences in the continuous electrocardiographic (ECG) criteria between Blacks and Whites were translated into a significantly higher (P < 0.05) prevalence of LVH in Black men when assessed by the Lewis and Sokolow–Lyon limb voltage criteria, the Sokolow–Lyon and Romhilt precordial voltage criteria, and the Sokolow–Lyon and Cornell voltage × duration products (Supplementary Table S2). Black as compared to White women had a lower (P < 0.05) prevalence of LVH when assessed by the Siegel total voltage sum, but a higher (P < 0.05) prevalence when categorized by the Sokolow–Lyon voltage-duration product. The prevalence of LVH defined using any of the 12 criteria was higher in Black as compared to White men (54.4% vs. 36.0%, P = 0.0006), but similar in Black and White women (18.9% vs. 16.5%, P = 0.57).
Association between ECG criteria for LVH and office and home SBP
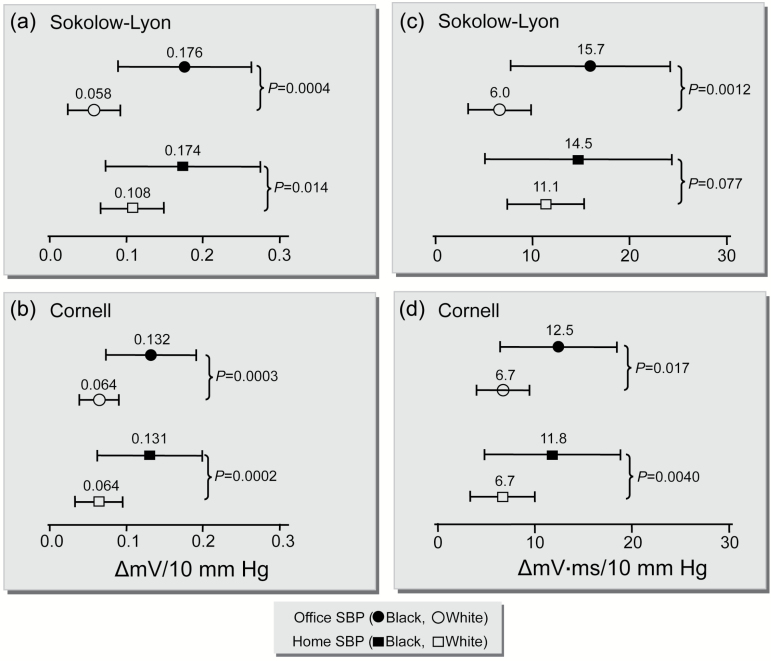
After adjustment for sex, age, body mass index, skinfold thickness, and antihypertensive drug intake, all ECG criteria based on precordial lead voltages (alone or in combination with limb-lead voltages) and the voltage-duration products were positively correlated (P < 0.01) with the office SBP in the 2 ethnicities. The slopes of these relations were significantly (P < 0.03) steeper in Blacks than in Whites (Table 4, Figure 2). In Blacks, a 10 mm Hg higher office SBP was associated with 0.18 mV (95% confidence interval [CI], 0.09–0.26; P < 0.0001) higher Sokolow–Lyon voltage, whereas in Whites this slope amounted to 0.06 mV per 10 mm Hg (CI, 0.02–0.09; P < 0.01). The ethnic difference was statistically significance (P < 0.001). Similarly, the increase in the Sokolow–Lyon voltage-product corresponding to a 10 mm Hg increase in the office SBP was 15.7 mV × ms (CI, 7.5–24.0; P < 0.0001) in Blacks and 6.0 (CI, 2.8–9.3; P < 0.0001) mV × ms in Whites (P for ethnic difference, 0.001). We observed the same trends for home SBP, although the ethnic differences did not reach statistical significance for the Wilson precordial voltage (P = 0.53) nor for the Sokolow–Lyon (P = 0.078) and Siegel (P = 0.32) voltage-duration products.
Table 4.
Multivariable-adjusted associations between ECG measurements and blood pressure by ethnicity
| ECG criteria (reference) | Office systolic pressure | Home systolic pressure | ||||
| Blacks | Whites | P | Blacks | Whites | P | |
| Limb-lead voltages, mV | ||||||
| Lewis3 | 0.022 (−0.045, 0.089) | 0.034 (−0.004, 0.073) | 0.011 | 0.064 (−0.013, 0.141) | −0.013 (−0.060, 0.033) | 0.58 |
| Gubner–Ungerleider3 | 0.033 (−0.015, 0.080) | 0.049 (0.028, 0.070)§ | 0.004 | 0.056 (0.001, 0.111)* | 0.034 (0.009, 0.060)† | 0.24 |
| Sokolow–Lyon12 | 0.019 (−0.007, 0.046) | 0.024 (0.012, 0.036)§ | 0.88 | 0.029 (−0.001, 0.059) | 0.017 (0.002, 0.031)* | 0.40 |
| Precordial lead voltages, mV | ||||||
| Sokolow–Lyon12 | 0.176 (0.089, 0.263)§ | 0.058 (0.023, 0.092)† | <0.001 | 0.174 (0.072, 0.275)‡ | 0.108 (0.067, 0.149)§ | 0.014 |
| Wilson13 | 0.084 (0.024, 0.144)† | 0.042 (0.019, 0.066)‡ | <0.0001 | 0.078 (0.008, 0.148)* | 0.077 (0.049, 0.105)§ | 0.53 |
| Romhilt14 | 0.191 (0.095, 0.287)‡ | 0.073 (0.038, 0.107)§ | <0.001 | 0.189 (0.077, 0.301)† | 0.139 (0.098, 0.180)§ | 0.006 |
| Combination of limb and precordial voltages, mV | ||||||
| Cornell15 | 0.132 (0.074, 0.190)§ | 0.064 (0.039, 0.090)§ | <0.001 | 0.131 (0.063, 0.199)‡ | 0.064 (0.033, 0.095)§ | < 0.001 |
| Siegel16 | 0.816 (0.440, 1.193)§ | 0.445 (0.286, 0.604)§ | 0.002 | 0.850 (0.413, 1.288)‡ | 0.721 (0.532, 0.910)§ | 0.029 |
| Manning17 | 0.194 (0.071, 0.317)† | 0.111 (0.057, 0.165)§ | 0.005 | 0.223 (0.082, 0.365)† | 0.207 (0.142, 0.271)§ | 0.028 |
| Voltages and QRS duration, mV.ms | ||||||
| Sokolow–Lyon18 | 15.7 (7.5, 24.0)‡ | 6.0 (2.8, 9.3)‡ | 0.001 | 14.5 (4.9, 24.1)† | 11.1 (7.2, 15.0)§ | 0.078 |
| Cornell19 | 12.5 (6.5, 18.4)§ | 6.7 (4.1, 9.4)§ | 0.002 | 11.8 (4.9, 18.8)‡ | 6.7 (3.4, 10.0)§ | 0.004 |
| Siegel19 | 74.5 (36.3, 113.2)‡ | 47.8 (29.6, 66.1)§ | 0.022 | 72.9 (28.1–117.7)† | 75.6 (53.8, 97.3)§ | 0.32 |
Values are regression coefficients (95% confidence interval) expressing the change in the ECG measurement associated with a 10-mm Hg increase in systolic blood pressure, adjusted for sex, age, body mass index, skinfold thickness, and intake of antihypertensive drugs. Significance of the associations: *P < 0.05; †P < 0.01; ‡P < 0.001; and §P < 0.0001. P values are for the ethnic differences in the associations.
Figure 2.
Multivariable-adjusted associations between the Sokolow–Lyon (a) and Cornell voltages (b) and the Sokolow–Lyon (c) and Cornell (d) voltage × duration products and systolic blood pressure on office or home measurements in Blacks and Whites. All associations were adjusted for sex, age, body mass index, skinfold thickness and intake of antihypertensive drugs. Changes (∆) in the voltages and voltage × duration products are given for a 10 mm Hg increase in systolic blood pressure (SBP). Horizontal bars denote the 95% confidence interval.
The limb-lead voltages were not consistently related to the office or home SBPs (Table 4). If anything, the relationship between the limb-lead voltage criteria as defined by Lewis and Gubner–Ungerleider were steeper in Whites than in Blacks (P < 0.05).
Association between the prevalence of LVH and office and home SBP
Table 5 shows the adjusted odds ratios for the associations between LVH analyzed on a dichotomous scale and both office and home SBPs. We did not calculate odds ratios for ECG criteria that identified less than 10 cases of LVH. For the Sokolow–Lyon precordial lead voltage and all the voltage-duration products, the odds ratios for office SBP were higher in Blacks than in Whites (P < 0.05). Using any of the 12 diagnostic criteria (Table 5), a 10 mm Hg higher office SBP was associated with a relative risk of LVH of 1.95 (CI, 1.47–2.60; P < 0.0001) in Blacks, while in Whites the odds ratio was 1.20 (CI, 1.06–1.35; P = 0.0038). The P value for the ethnic difference was <0.0001. The same trends were observed for home SBP. When assessing LVH by any of the 12 criteria, a 10-mm Hg higher home SBP was associated with an odds ratio of 2.27 (CI, 1.61–3.19; P < 0.0001) in Blacks and 1.30 (CI, 1.12–1.51; P = 0.0006) in Whites (P for ethnic difference < 0.0001).
Table 5.
Multivariable-adjusted odds ratios for having left ventricular hypertrophy in relation to systolic blood pressure
| Definition of LVH (reference) | Office systolic pressure | Home systolic pressure | ||||
| Blacks | Whites | P | Blacks | Whites | P | |
| Limb-lead voltages | ||||||
| Lewis3 | 1.06 (0.74, 1.54) | 1.14 (0.84, 1.54) | 0.73 | 1.18 (0.76, 1.83) | 1.11 (0.75, 1.63) | 0.41 |
| Gubner–Ungerleider3 | (3) | (7) | (3) | (7) | ||
| Sokolow–Lyon12 | (6) | (7) | (6) | (7) | ||
| Precordial lead voltages | ||||||
| Sokolow–Lyon12 | 1.57 (1.19, 2.06)† | 1.17 (0.93, 1.46) | 0.030 | 1.58 (1.15, 2.17)† | 1.75 (1.31,2.33)§ | 0.59 |
| Wilson13 | (3) | (3) | (3) | (3) | ||
| Romhilt14 | 1.58 (1.07, 2.33)* | (7) | 1.34 (0.84, 2.12) | (7) | ||
| Combination of limb and precordial voltages | ||||||
| Cornell15 | (8) | 1.18 (0.84, 1.67) | (8) | 0.96 (0.62, 1.49) | ||
| Siegel16 | 1.72 (1.28, 2.32)‡ | 1.40 (1.21, 1.62)§ | 0.067 | 1.68 (1.21, 2.33)† | 1.48 (1.23, 1.77)§ | 0.10 |
| Manning17 | (4) | 1.40 (0.75, 2.61) | (4) | 1.96 (0.88, 4.34) | ||
| Voltage × QRS duration products, mV.ms | ||||||
| Sokolow–Lyon18 | 2.21 (1.53, 3.18)§ | 1.15 (0.97, 1.36) | 0.003 | 2.21 (1.53, 3.18)§ | 1.52 (1.22, 1.89)‡ | 0.09 |
| Cornell19 | 1.92 (1.44, 2.56)§ | 1.15 (0.97, 1.36) | 0.003 | 1.61 (1.12, 2.31)† | 1.14 (0.85, 1.52) | 0.03 |
| Siegel19 | 1.65 (1.19, 2.28)† | 1.21 (0.96, 1.53) | 0.037 | 1.48 (1.07, 2.05)* | 1.47 (1.20, 1.80)‡ | 0.66 |
| Any of the criteria | 1.95 (1.47, 2.60)§ | 1.20 (1.06, 1.35)† | <0.0001 | 2.27 (1.61, 3.19)§ | 1.30 (1.12, 1.51)‡ | < 0.0001 |
Values are odds ratios (95% confidence interval) associated with a 10-mm Hg increase in systolic pressure and were adjusted for sex, age, body mass index, skinfold thickness, and intake of antihypertensive drugs. Significance of the odds ratios: *P < 0.05; †P < 0.01; ‡P < 0.001; and §P < 0.0001. If the number of patients with left ventricular hypertrophy was smaller than 10 (number given), the odds ratio was not calculated. P values are for the ethnic differences in the odds ratios.
DISCUSSION
We assessed the association of various ECG measurements commonly applied for the diagnosis of LVH with SBP on office and home measurement in Black Nigerians and White Flemish recruited from the population with as objective to search for ethnic differences. The key findings can be summarized as follows: (i) most QRS voltage combinations and voltage-duration products tended to be higher in Blacks than in Whites, irrespective of sex, age, and other confounding variables; (ii) in both ethnicities, the precordial QRS voltage sums, combined or not with the limb voltages, and the voltage-duration products were positively associated with both office and home SBP; (iii) the slope of these associations were up to 3 times steeper in Blacks than in Whites; (iv) and these results were confirmed when LVH was analyzed on a dichotomous rather than a continuous scale.
Our results are in agreement with previous studies6–9 showing that left ventricular mass increases with BP in both Blacks and Whites, but more sharply in Blacks than in Whites. However, the Black participants included in these studies were Americans of African ancestry.6–9 The potential racial disparity in the cardiac adaptation to high BP has not yet been studied in Black populations born and living in Africa. None of the aforementioned studies assessed consistency between the findings based on the in-office and the out-of-the office BP.6–9 In addition, previous studies assessed LVH, using echocardiography7,29,30 or electrocardiography calibrated against echocardiography.7 We assessed LVH using various published combinations of ECG voltages and QRS duration (Table 13,12–19). Electrocardiography is less sensitive for the detection of anatomically increased left ventricular mass as compared to echocardiography7,29,30 and other cardiac imaging modalities.10 However, electrocardiography might provide separate information on myocardial integrity and neurohumoral and/or biochemical changes in the myocardium that cannot be detected using echocardiography.11 Calibrating electrocardiography against echocardiography is therefore incorrect, but unfortunately, this practice has been perpetuated in the medical literature over the years since the advent of echocardiography. In a recent report, Bacharova and colleagues5 compared the prognostic value of LVH diagnosed by electrocardiography and magnetic resonance imaging in 4,748 participants enrolled in the Multi-Ethnic Study of Atherosclerosis. They observed that LVH detected by ECG (hazard ratio, 1.51; CI, 1.03–2.20) and LVH diagnosed with magnetic resonance imaging (hazard ratio, 1.81; CI, 1.33–2.46) were equally predictive of cardiovascular events. These findings confirm that LVH defined using electrocardiography and magnetic resonance imaging, although being different phenotypes, both carry important prognostic information.5
To our knowledge, no previous report has assessed the ethnic differences in the effect of BP on QRS voltages or voltage-duration products as continuous variables (Table 4), whereas most considered ECG-LVH (Table 5). Categorizing an outcome has several limitations. First, LVH as a binary variable, compared with a continuous outcome, removes phenotypic variation. Second, most of the ECG criteria currently in use were developed in patients who had clinical conditions like severe hypertension15 or aortic stenosis16 that were expected to cause hypertrophy, were calibrated against autopsy findings of an enlarged heart,12,15,16,31,32 or against left ventricular mass determined by echocardiography7 or magnetic resonance imaging.10 The implication is that published ECG criteria for LVH (Table 13,12–19) define a phenotype among patients, who are already at the terminal stage of the cardiovascular disease continuum. Moreover, some of those criteria were developed over more than 2 decades ago when the thresholds for the diagnosis of hypertension were 160 mm Hg systolic or 100 mm Hg diastolic. We reasoned that increasing BP may have an incremental and graded effect on ECG voltages and voltage-duration products, which probably starts at much lower levels of BP than used for the development of the published criteria (Table 13,12–19).
If our findings are confirmed, they will help clinicians not only for risk assessment of patients within a large spectrum of cardiovascular risk, but also to monitor antihypertensive therapy. In the double-blind placebo-controlled Systolic Hypertension in Europe Trial, we defined LVH prospectively as the sum of 3 voltages (R in aVL, S in V1, and R in V5) both at baseline and at yearly follow-up visits.33 The adjusted relative hazard ratio associated with a 1 mV higher voltage sum at baseline, amounted to 1.10 (CI, 1.02–1.18) and 1.15 (CI, 1.04–1.27) for all-cause and cardiovascular mortality and to 1.21 (CI, 1.08–1.36) and 1.18 (CI, 1.08–1.29) for stroke and cardiac events, respectively (P < 0.01). A 1-mV decrease in ECG voltages during follow-up independently predicted a lower incidence of cardiac events with a hazard ratio of 0.86 (CI, 0.76–0.98); P = 0.05), but not of stroke or mortality.33
The prognostic implication of electrocardiographically derived LVH among Blacks born and living in Africa is not yet known. However, prospective studies in African Americans suggest that LVH contributes more to the risk of cardiovascular mortality in Blacks than in Whites.34,35 Among 1,089 Black people drawn from a Chicago hospital registry Liao and colleagues34 studied the effect of echocardiographically determined LVH on survival in comparison with the number of stenotic coronary vessels and left ventricular systolic function. In a multivariable analysis, the relative risk of death associated with LVH was 2.4 as compared to 1.6 and 2.0 for multivessel disease and ejection fraction lower than 45%. The population attributable risk fraction in this cohort revealed that for every 100 deaths 1%, 9%, 22%, and 37% were attributable to single-vessel disease, left ventricular systolic dysfunction, multivessel disease, and LVH, respectively.34
Our current findings are particularly relevant for low- and middle-income countries, where health care resources are limited and the infrastructure and skills to operate high-tech equipment, such as sophisticated echocardiographic devices or cardiac magnetic resonance imaging are lacking. The implication is that assessment of electrocardiographically derived LVH should be an integral part of the cardiovascular assessment even when patients present with BP within the ranges presumed to be normal.
Notwithstanding the clinical relevance of our current observations, our study should also be interpreted within the context of its potential limitations and applied methodologic approaches. We considered only 12 among 37 criteria36 that have been reported in the literature. However, these 12 selected criteria3,12–19 are among those most commonly used in the literature and in clinical practice. Furthermore, we combined criteria whose diagnostic performances have been compared between Blacks and Whites or evaluated for diagnostic performance among Nigerians.37 We also decided to use variables that can easily be obtained from automated electrocardiography without the need for interpretation by a cardiologist. This is to ensure widespread clinical applicability of our methods in a setting, where expertise for reading electrocardiograms is scarce. Our study involved a small sample of Nigerians living in Abuja and may not be representative for the whole sub-Saharan continent. However, our results have external validity as our findings are in line with other findings among Blacks living in the United States.6–9 Additionally, the same validated epidemiologic methods were used in both population cohorts compared in this report.
Perspectives
We showed strong association between ECG-LVH and SBP in Blacks born and living in sub-Saharan Africa. While confirming previous findings in African compared with White Americans,6–9 we advocate that our observations are particularly relevant for the low-resource sub-Saharan region. Portable ECG devices, which as the recorder used in the current study have the size of a small pocket book and do not require a power module and which via smartphone, notebook or an integrated SIM card (subscriber identity module) can access internet might become a practicable instrument for risk stratification in remote or deprived African communities. Combined with office and home BP, the ECG indexes might be logged onto a central server, via which experienced ECG readers and hypertension specialist might assist local health care providers in guiding the management of cardiovascular risk.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension online.
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the expert clerical assistance of Mrs. Vera De Leebeeck and Mrs. Renilde Wolfs (Studies Coordinating Centre, Leuven, Belgium). The European Union (HEALTH-FP7-278249-EUMASCARA, HEALTH-F7-305507 HOMAGE and the European Research Council (Advanced Researcher Grant 2011-294713-EPLORE and Proof-of-Concept Grant 713601-uPROPHET) and the Fonds voor Wetenschappelijk Onderzoek Vlaanderen, Ministry of the Flemish Community, Brussels, Belgium (G.0881.13, G.088013, and 11Z0916N) currently support the Studies Coordinating Centre in Leuven.
References
- 1. Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. Arch Intern Med 1993; 153:598–615. [DOI] [PubMed] [Google Scholar]
- 2. Sullivan JM, Vander Zwaag RV, el-Zeky F, Ramanathan KB, Mirvis DM. Left ventricular hypertrophy: effect on survival. J Am Coll Cardiol 1993; 22:508–513. [DOI] [PubMed] [Google Scholar]
- 3. Hsieh BP, Pham MX, Froelicher VF. Prognostic value of electrocardiographic criteria for left ventricular hypertrophy. Am Heart J 2005; 150:161–167. [DOI] [PubMed] [Google Scholar]
- 4. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990; 322:1561–1566. [DOI] [PubMed] [Google Scholar]
- 5. Bacharova L, Chen H, Estes EH, Mateasik A, Bluemke DA, Lima JA, Burke GL, Soliman EZ. Determinants of discrepancies in detection and comparison of the prognostic significance of left ventricular hypertrophy by electrocardiogram and cardiac magnetic resonance imaging. Am J Cardiol 2015; 115:515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arnett DK, Rautaharju P, Crow R, Folsom AR, Ekelund LG, Hutchinson R, Tyroler HA, Heiss G; The ARIC Investigators Black-White differences in electrocardiographic left ventricular mass and its association with blood pressure (the ARIC Study). Am J Cardiol 1994; 74:257–252. [DOI] [PubMed] [Google Scholar]
- 7. Arnett DK, Rautaharju P, Sutherland S, Usher B, Keil J. Validity of electrocardiographic estimates of left ventricular hypertrophy and mass in African Americans (The Charleston Heart Study). Am J Cardiol 1997; 79:1289–1292. [DOI] [PubMed] [Google Scholar]
- 8. Lorber R, Gidding SS, Daviglus ML, Colangelo LA, Liu K, Gardin JM. Influence of systolic blood pressure and body mass index on left ventricular structure in healthy African-American and White young adults: the CARDIA study. J Am Coll Cardiol 2003; 41:955–960. [DOI] [PubMed] [Google Scholar]
- 9. Fox E, Taylor H, Andrew M, Han H, Mohamed E, Garrison R, Skelton T. Body mass index and blood pressure influences on left ventricular mass and geometry in African Americans: the Atherosclerotic Risk In Communities (ARIC) Study. Hypertension 2004; 44:55–60. [DOI] [PubMed] [Google Scholar]
- 10. Jain A, Tandri H, Dalal D, Chahal H, Soliman EZ, Prineas RJ, Folsom AR, Lima JAC, Bluemke DA. Diagnostic and prognostic utility of ECG for left ventricular hypertrophy defined by MRI in relationship to ethnicity: The Multi-Ethnic Study of Atherosclerosis (MESA). Am Heart J. 2010; 159:652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bacharova L, Estes EH, Bang LE, Hill JA, Macfarlane PW, Rowlandson I, Schillaci G. Second statement of the working group on electrocardiographic diagnosis of left ventricular hypertrophy. J Electrocardiol 2011; 44:568–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J 1949; 37:161–186. [DOI] [PubMed] [Google Scholar]
- 13. Wilson FN, Johnston FD, Rosenbaum FF, Erlanger H, Kossmann CE, Hecht H, Cotrim N, Menezes de Oliveira R, Scarsi R, Barker PS. The precordial electrocardiogram. Am Heart J 1943; 29:19–85. [Google Scholar]
- 14. Romhilt DW, Bove KE, Norris RJ, Conyers E, Conradi S, Rowlands DT, Scott RC. A critical appraisal of the electrocardiographic criteria for the diagnosis of left ventricular hypertrophy. Circulation 1969; 40:185–195. [DOI] [PubMed] [Google Scholar]
- 15. Casale PN, Devereux RB, Alonso DR, Campo E, Kligfield P. Improved sex-specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: validation with autopsy findings. Circulation 1987; 75:565–572. [DOI] [PubMed] [Google Scholar]
- 16. Siegel RJ, Roberts WC. Electrocardiographic observations in severe aortic valve stenosis: correlative necropsy study to clinical, hemodynamic, and ECG variables demonstrating relation of 12-lead QRS amplitude to peak systolic transaortic pressure gradient. Am Heart J 1982; 103:210–221. [DOI] [PubMed] [Google Scholar]
- 17. Manning GW, Smiley JR. QRS-voltage criteria for left ventricular hypertrophy in a normal male population. Circulation 1964; 29:224–230. [DOI] [PubMed] [Google Scholar]
- 18. Hudkins KL, Giachelli CM, Cui Y, Couser WG, Johnson RJ, Alpers CE. Osteopontin expression in fetal and mature human kidney. J Am Soc Nephrol 1999; 10:444–457. [DOI] [PubMed] [Google Scholar]
- 19. Molloy TJ, Okin PM, Devereux RB, Kligfield P. Electrocardiographic detection of left ventricular hypertrophy by the simple QRS voltage-duration product. J Am Coll Cardiol 1992; 20:1180–1186. [DOI] [PubMed] [Google Scholar]
- 20. World Medical Association. Declaration of Helsinki. J Am Med Ass 2013; 227:184–189. [Google Scholar]
- 21. Odili AN, Ogedengbe JO, Nwegbu M, Anumah FO, Asala S, Staessen JA. Nigerian Population Research on Environment, Gene and Health (NIPREGH) - objectives and protocol. J Biomed Res 2014; 28:360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu YP, Gu YM, Thijs L, Knapen MH, Salvi E, Citterio L, Petit T, Carpini SD, Zhang Z, Jacobs L, Jin Y, Barlassina C, Manunta P, Kuznetsova T, Verhamme P, Struijker-Boudier HA, Cusi D, Vermeer C, Staessen JA. Inactive matrix Gla protein is causally related to adverse health outcomes: a Mendelian randomization study in a Flemish population. Hypertension 2015; 65:463–470. [DOI] [PubMed] [Google Scholar]
- 23. Kligfield P, Gettes LS, Bailey JJ, Childers R, Deal BJ, Hancock W, van Herpen G, Kors JA, Macfarlane P, Mirvis DM, Pahlm O, Rautaharju P, Wagner GS. Recommendations for the standardization and interpretation of the electrocardiogram. Part I: the electrocardiogram and its technology. A scientific statement from the American Heart Associatione Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorese by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 2007; 49:1109–1127. [DOI] [PubMed] [Google Scholar]
- 24. M’Buyamba-Kabangu JR, Anisiuba BC, Ndiaye MB, Lemogoum D, Jacobs L, Ijoma CK, Thijs L, Boombhi HJ, Kaptue J, Kolo PM, Mipinda JB, Osakwe CE, Odili A, Ezeala-Adikaibe B, Kingue S, Omotoso BA, Ba SA, Ulasi II, Staessen JA; Newer versus Older Antihypertensive Agents in African Hypertensive Patients Trial (NOAAH) Investigators Efficacy of newer versus older antihypertensive drugs in Black patients living in sub-Saharan Africa. J Hum Hypertens 2013; 27:729–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O’Brien E, Asmar R, Beilin L, Imai Y, Mancia G, Mengden T, Myers M, Padfield P, Palatini P, Parati G, Pickering T, Redon J, Staessen J, Stergiou G, Verdecchia P; European Society of Hypertension Working Group on Blood Pressure Monitoring Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self blood pressure measurement. J Hypertens 2005; 23:697–701. [DOI] [PubMed] [Google Scholar]
- 26. Coleman A, Freeman P, Steel S, Shennan A. Validation of the Omron 705IT (HEM-759-E) oscillometric blood pressure monitoring device according to the British Hypertension Society protocol. Blood Press Monit 2006; 11:27–32. [DOI] [PubMed] [Google Scholar]
- 27. Topouchian J, Agnoletti D, Blacher J, Youssef A, Ibanez I, Khabouth J, Khawaja S, Beaino L, Asmar R. Validation of four automatic devices for self-measurement of blood pressure according to the international protocol of the European Society of Hypertension. Vasc Health Risk Manag 2011; 7:709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003; 26 (Suppl 1):S5–S20. [DOI] [PubMed] [Google Scholar]
- 29. Chaturvedi N, Athanassopoulos G, McKeigue PM, Marmot MG, Nihoyannopoulos P. Echocardiographic measures of left ventricular structure and their relation with rest and ambulatory blood pressure in Blacks and Whites in the United Kingdom. J Am Coll Cardiol 1994; 24:1499–1505. [DOI] [PubMed] [Google Scholar]
- 30. Chapman JN, Mayet J, Chang CL, Foale RA, Thom SA, Poulter NR. Ethnic differences in the identification of left ventricular hypertrophy in the hypertensive patient. Am J Hypertens 1999; 12:437–442. [DOI] [PubMed] [Google Scholar]
- 31. Herrmann GR, Wilson FN. Ventricular hypertrophy—a comparison of electrocardiographic and post-mortem observations. Heart 1922; 9:91–147. [Google Scholar]
- 32. Wilson FN. The distribution of the potential differences produced by the heart beat within the body and at its surface. Am Heart J 1930; 5:599–616. [Google Scholar]
- 33. Fagard RH, Staessen JA, Thijs L, Celis H, Birkenhäger WH, Bulpitt CJ, de Leeuw PW, Leonetti G, Sarti C, Tuomilehto J, Webster J, Yodfat Y; Systolic Hypertension in Europe (Syst-Eur) Trial Investigators Prognostic significance of electrocardiographic voltages and their serial changes in elderly with systolic hypertension. Hypertension 2004; 44:459–464. [DOI] [PubMed] [Google Scholar]
- 34. Liao Y, Cooper RS, McGee DL, Mensah GA, Ghali JK. The relative effects of left ventricular hypertrophy, coronary artery disease, and ventricular dysfunction on survival among Black adults. JAMA 1995; 273:1592–1597. [PubMed] [Google Scholar]
- 35. Havranek EP, Froshaug DB, Emserman CD, Hanratty R, Krantz MJ, Masoudi FA, Dickinson LM, Steiner JF. Left ventricular hypertrophy and cardiovascular mortality by race and ethnicity. Am J Med 2008; 121:870–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hancock WE, Deal BJ, Mirvis DM, Okin P, Kligfield P, Gettes LS. AHA/ACCF/HRS recommendations for the standardization of interpretation of the electrocardiogram. J Am Coll Cardiol 2009; 53:992–1002. [DOI] [PubMed] [Google Scholar]
- 37. Dada A, Adebiyi AA, Aje A, Oladapo OO, Falase AO. Standard electrocardiographic criteria for left ventricular hypertrophy in Nigerian hypertensives. Ethn Dis 2005; 15:578–584. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.