Figure 3.
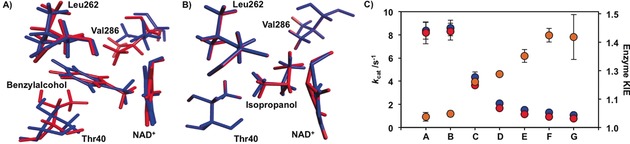
A, B) Normal mode associated to the substituents bending coupled to the hydride transfer in BsADH displayed as a superposition of two structures of the active site (red and blue) with benzyl alcohol (A) and isopropanol (B). C) The steady‐state rate constants (k cat) for “light” (blue) and “heavy” (red) BsADH, and enzyme kinetic isotope effect (orange) from substrate A–G at 20 °C and pH 7.0. The substrates are: isopropanol (A), 2‐butanol (B), ethanol (C), 1‐pentanol (D), cyclopentanol (E), and cinnamyl (F) and benzyl (G) alcohols.
