Abstract
Background
The number of obese pediatric patients requiring anesthesia is rapidly increasing. Although fentanyl is a commonly used narcotic during surgery, there is no pharmacokinetic (PK) data available for optimal dosing of fentanyl in adolescents with clinically severe obesity.
Materials and Methods
An IRB-approved exploratory pilot study was conducted in 6 adolescents aged 14 to 19 years undergoing bariatric surgery. Mean total body weight (TBW) and mean BMI were 137.4 ± 14.3 kg, and 49.6 ± 6.4 kg/m2 (99.5th BMI percentile), respectively. Fentanyl was dosed intravenously for intraoperative analgesia based on ideal body weight per standard of care. PK blood samples were drawn over a 24 hour post-dose period. Fentanyl PK parameters were calculated by non-compartmental analysis.
Results
Mean fentanyl AUC0–∞ was 1.5 ± 0.5 h*ng/mL. Systemic clearance of fentanyl was 1522 ± 310 mL/min and 11.2 ± 2.6 mL/min*kg TBW. Volume of distribution was 635 ± 282 L and 4.7 ± 2.1 L/kg TBW. While absolute clearance was increased, absolute volume of distribution was comparable to previously established adult values.
Conclusions
These results suggest that fentanyl clearance is enhanced in adolescents with clinically severe obesity while volume of distribution is comparable to previously published studies.
1. Introduction and Background
Obesity represents a rapidly growing public health issue across the globe. Because obesity is often accompanied by co-morbid diseases, pharmacotherapy and/or surgical intervention may frequently be necessary.
Bariatric surgery is an option for carefully selected adolescents with clinically severe obesity who are unable to lose weight by conventional standards. Unfortunately, there is a lack of precision in the dosing of drugs for this special patient population. Moreover, the effective use of anesthetic medications during bariatric surgery is essential to ensure patient safety.
Fentanyl is a commonly used synthetic opioid used to manage moderate to severe pain in adolescent patients. It has a rapid onset and short duration of action. Fentanyl is mainly metabolized by cytochrome P450 3A (CYP3A) to norfentanyl [1] and shows dose-independent pharmacokinetics [2]. Fentanyl has been described as a high-extraction ratio drug and its elimination is dependent on hepatic blood flow [3]. All known metabolites of fentanyl are pharmacologically inactive and up to 76% of a single dose is excreted in the urine (approximately 6.5% remains unchanged) [4].
Lipophilic drugs like fentanyl are primarily distributed into body fat presumably resulting in an increased volume of distribution in obese patients. Different weight descriptors, for example total body weight (TBW), ideal body weight (IBW), lean body weight (LBW) and/or pharmacokinetic mass (PKM) are currently being used to determine the required dose of fentanyl in obese patients [5, 6]. Dosing fentanyl based upon LBW or IBW may avoid overdosing and deleterious side effects such as postoperative respiratory depression.
The pharmacokinetics (PK) of fentanyl in adults has been well documented and shows a high inter-individual variability. Studies in infants and children have reported age-dependent differences in the PK and pharmacodynamic parameters of intravenous fentanyl with an enhanced clearance particularly during the first years of life [7–10].
The purpose of this exploratory pilot study was to investigate the PK of fentanyl in adolescent patients with clinically severe obesity undergoing bariatric surgery.
2. Materials and Methods
This prospective, single-center pilot study was approved by the Institutional Review Board at Children’s National Health System and was registered with clinicaltrials.gov (NCT01955993). Written informed consent and assent were obtained from all study participants or their parents (if applicable) before any study measures were initiated.
2.1 Study population
Adolescent patients diagnosed with clinically severe obesity (BMI > 99th percentile) between 14 and 19 years of age with an American Society of Anesthesiology (ASA) classification of I, II or III were eligible for participation. Each patient received fentanyl per standard of care during the perioperative period. All patients were admitted to the hospital postoperatively for at least 24 hours observation according to the surgical plan of care.
Exclusion criteria were pregnancy, lactation, hypersensitivity to any opioid, prior exposure to any opioid including fentanyl within a 24 hour period and treatment with inhibitors or inducers of cytochrome CYP3A within two weeks before inclusion.
2.2 Study Design and Procedures
After an overnight fast of at least 8 hours, a peripheral intravenous catheter was placed in a forearm vein. If necessary, 2 mg midazolam (midazolam hydrochloride, Baxter, Deerfield, IL, USA) was administered for anxiolysis within 5 minutes before fentanyl. General anesthesia was induced using propofol (1–3 mg/kg of TBW; 1%-emulsion, Fresenius Kabi, Lake Zurich, IL, USA), fentanyl (fentanyl citrate, Hospira, Lake Forest, IL, USA), and succinylcholine (1mg/kg per TBW; succinylcholine chloride, Hospira), facilitating rapid and reliable intubation. General anesthesia was maintained with desflurane (Baxter) and rocuronium (rocuronium bromide, Fresenius Kabi) during the procedure. The induction dose of fentanyl was administered based on IBW (1–2 mcg per kg IBW) using the formula [45.4 kg + 0.89 x (height in cm - 152.4)] for female patients [5]. Using IBW for fentanyl dosing is the standard of care for obese patients undergoing bariatric anesthesia at our institution. Additional doses of fentanyl could be given if considered necessary by the responsible anesthesiologist.
A second peripheral intravenous catheter on the opposite forearm was inserted to facilitate adequate fluid hydration, and also used for blood sampling. Standard monitoring according to the ASA’s guidelines was employed during general anesthesia, including noninvasive blood pressure measurements, 3-lead electrocardiogram, continuous pulse oximetry, and capnography.
Intraoperative pain management included intravenous acetaminophen (500–650 mg, Ofirmev, acetaminophen, solution for injection, Cadence Pharmaceuticals, San Diego, CA, USA) and intravenous ketorolac (30 mg, ketorolac tromethamine, Fresenius Kabi) approximately one hour prior to extubation.
Perioperative intravenous antibiotics (2 g cefazolin) and antiemetics (4–6 mg ondansetron) were administered per routine management. Reversal of neuromuscular blockade was achieved with neostigmine and glycopyrrolate. Tracheal extubation occurred with each patient wide-awake. Postoperative analgesia was provided by 2mg morphine only as needed (morphine sulfate, Hospira), 500–650mg acetaminophen (every 8 hours), and 30 mg ketorolac (every 8 hours), each prescribed around the clock and administered intravenously.
Clinical data were collected from the electronic medical record (Cerner Corporation, North Kansas City, MO, USA) including medical history, physical examination, pre- and postoperative laboratory studies, and perioperative administered medications.
2.3 Blood Sampling
Ten timed blood samples (1.5 mL each) were drawn into heparinized vacutainer tubes (BD Diagnostics, Franklin Lakes, NJ, USA) immediately before fentanyl injection at 5, 15 and 30 minutes as well as 1, 2, 4, 6, 12 and 24 hours after fentanyl administration. If additional doses of fentanyl were required for pain management, blood samples of 1.5 mL were withdrawn before each fentanyl dosing. Plasma was separated from whole blood by centrifugation (2000 g, 10 minutes at 4 °C) and stored at −70 °C until analysis.
2.4 Fentanyl Assay
Plasma concentrations of fentanyl were determined by validated assays as previously published [11]. The lower limit of quantification was 0.05 ng/mL (calibration range: 0.05–10.0 ng/mL). Analytical bias, imprecision and inaccuracy was <15 %.
2.5 Pharmacokinetic and Statistical Analysis
Due to the exploratory nature of this study, no sample size calculation was performed. Pharmacokinetic parameters of fentanyl were calculated using noncompartmental analysis (Kinetica 5.0, ThermoFisher Scientific, Waltham, MA, USA). Apparent volume of distribution was calculated based on area under the curve. Previously established PK profiles in children [8, 10, 12] were used for comparison. Statistical analyses between groups were conducted using an unpaired t-test with Welch’s correction (Prism 6.00, GraphPad Software Inc., La Jolla, CA, USA). A p-value <0.05 was considered to be significant.
3. Results
3.1 Study population
Six female adolescent patients aged 14 to 19 years (mean 16.6 years) undergoing laparoscopic sleeve gastrectomy for treatment of their clinically severe obesity were included in this pilot study. Patient characteristics are provided in table 1. Total body weight ranged from 117.8 to 154.6 kg (mean 137.4 kg), ideal body weight ranged from 53.1 to 62.0 kg (mean 58.0 kg) and BMI ranged from 43.0 to 59.6 kg/m2 (mean 49.6 kg/m2, 99.5th percentile). Ethnicities included three Caucasian patients, one African American, one Caucasian-Hispanic and one African American-Hispanic. Age and body weight were significantly different when compared to previously published pediatric control groups (see table 1). All patients were assigned an ASA status of II or III.
Table 1.
Fentanyl pharmacokinetics in adolescents with clinically severe obesity compared to children [8, 10] and obese adults [6].
| Parameter | Severely obese adolescents | Johnson et al. [10] 10–14 yr |
Katz et al. [8] > 6 yr |
Shibutani et al. [6] obese adults |
Shibutani et al. [6] lean adults |
|---|---|---|---|---|---|
| Clinical setting | bariatric surgery | surgery | mechanical ventilation due to critical illness | postoperative analgesia after major elective surgery | |
| ASA category | II or III | I or II | n.a. | III (median) | III (median) |
| n | 6 | 3 | 5 | 10 | 16 |
| Age (yr) | 16.6 ± 1.5 | 12 ± 1.7 * | 9.5 ± 2.8 ** | 47 ± 16 *** (n=39) | 60 ± 16 *** (n=70) |
| TBW (kg) | 137.4 ± 14.3 | 58.3 ± 13.3 *** | 117.3 ± 33.4 n.s. |
68.5 ± 9.5 *** | |
| Dose (μg) | 138 ± 49 | 253 ± 71 a) | |||
| Dose (μg/kg TBW) | 1.0 ± 0.4 | 4.34 ± 1.22 * | 5 (bolus), 0.47– 6.2/h (range) | 1–2 (bolus), 0.5–4.2/h (range) | |
| Mode of administration | bolus dose | bolus dose (2–10 minute infusion) | bolus dose, followed by continuous infusion (steady state) | bolus, followed by continuous infusion (steady state) | |
| Sampling time (h) | up to 24 h | up to 10 h | up to 48 hours post infusion | 24.4 h | 24.6 h |
| CL (mL/min* kg TBW) | 11.2 ± 2.6 | 7.1 ± 1.2 * | 8.0 ± 3.9 n.s. | 8.76 ± 1.74 n.s. | 10.48 ± 1.81 n.s. |
| CL (mL/min) | 1522 ± 310 | 414 ± 70 a) | 986 ± 155 ** | 718 ± 163 *** | |
| Vd (L/kg TBW) | 4.7 ± 2.1 | 1.9 ± 1.0 * | |||
| Vd (L) | 635 ± 282 | 111 ± 61 a) | |||
| t1/2 (h) | 4.8 ± 2.0 | 3.5 ± 1.2 n.s. | |||
p compared with the present study using an unpaired t test with Welch’s correction:
p<0.05,
p<0.01,
p<0.001,
calculated based on mean TBW, not included into statistical analysis.
Abbreviations: TBW = total body weight, CL = total body clearance, t1/2 = terminal elimination half-life, Vd = volume of distribution, n.a. = not applicable, n.s. = not significant.
Comorbidities included obstructive sleep apnea (OSA), polycystic ovarian syndrome, insulin resistance / diabetes mellitus type 2, hypertension, hypercholesterolemia, asthma and hypothyroidism, non-alcoholic steatohepatitis (NASH), gastro esophageal reflux, attention deficit hyperactivity disorder and depression.
Co-medications were levothyroxine (n=1), metformin (n=2), lisinopril (n=1), amlodipine (n=1), fluticasone (n=1), salbutamol (n=1), venlafaxine (n=1) and lisdexamfetamine (n=1). One patient had no medications. There were no known drug interactions between fentanyl and these compounds.
3.2 Pharmacokinetics of fentanyl
Individual total fentanyl dosage ranged from 50 to 200 mcg resulting in a total fentanyl dose of 1.0 ± 0.4 μg/kg body weight. Fentanyl PK parameters are summarized in table 1. Fentanyl total body clearance was 1522 ± 310 mL/min, or 11.2 ± 2.6 mL/kg/min, when normalized to total body weight. Fentanyl volume of distribution was 635 ± 282 L, or 4.7 ± 2.1 L/kg, when normalized to weight. Table 1 also shows previously assessed fentanyl pharmacokinetics in non-obese children as well as obese and lean adults for comparative purpose.
3.3 Efficacy
Five of the six studied patients needed additional opioids during their surgical procedure, whereas all six patients received acetaminophen and ketolorac during their postoperative recovery for pain control.
3.4 Safety
All patients underwent general anesthesia and laparoscopic surgery successfully without complications. Routine laboratory analyses had shown normal renal function in all patients and normal liver enzymes in all but one patient, who presented with elevated ALT and AST (within 2 x of the upper limit of normal) and had been prediagnosed with metabolic syndrome. INR and bilirubin were within normal ranges in all patients.
4. Discussion
4.1 Fentanyl Pharmacokinetics
This exploratory pilot study aims to investigate the PK of fentanyl in a population of adolescents with clinically severe obesity. There is no doubt concerning normal-weight age-matched controls representing an optimal comparison group for this study. However, since most surgeries in otherwise healthy adolescents in that age range are performed as outpatient procedures, it was not possible to obtain samples over 24 hours from a sufficient number of healthy lean controls. In addition, the rates of childhood overweight and obesity are rising in the US [13], resulting in an even smaller number of potentially recruitable normal-weight patients.
Nevertheless, even with the small number of obese patients in this pilot study we could demonstrate that fentanyl total body clearance (in mL/min) is enhanced when compared to adult and pediatric data from the literature (table 1). Obese patients who were otherwise healthy showed an increased circulating blood volume, not only due to an increase of the total blood volume, but also due to an increased cardiac output [14]. It has also been reported that splanchnic blood flow in obese patients is increased by up to 20% [14].
Johnson et al. [10] studied the pharmacokinetics of fentanyl in children aged 10 to 14 years undergoing surgery (table 1). Reported absolute and normalized clearance values were clearly lower than those measured in our study, which may be due to the difference in body weight of these patients who received fentanyl over a 2–10 minute infusion before undergoing surgery compared to our study cohort. While there was no relevant difference in elimination half-life, the significant difference in volume of distribution between the obese adolescents and the children studied by Johnson et al. could be attributed to the higher body fat mass in the obese adolescents. The extent of the volume of distribution, however, does not always correlate with the degree of lipophilicity of a drug [15].
Katz et al. [8] also found lower values than the current study (8.0 ± 3.9 mL/min*kg vs. 11.2 ± 2.6 mL/min*kg) even when expressed per kg bodyweight though not statistically significant (p=0.13) which may be due to the considerable variability in critically ill children receiving a continuous fentanyl infusion over 7 to 136 hours. The patients’ body weights were unfortunately not available for that study so the calculation of the absolute clearance was not possible. Assuming that the patients’ body weights were within the normal range, their absolute fentanyl clearance would be lower than in our study. Freid et al. observed clearance values (mean ± SD 19.3 ± 12.4 mL/kg/min) comparable to adult data in children aged one day to 10.9 years, but unfortunately the patients’ age distribution and body weights were not specified [12].
Other factors which may influence the calculation of fentanyl PK, especially its elimination half-life are whether fentanyl was administered as a bolus dose (in our study) or continuous infusion and the length of the blood sampling scheme. The dosing scheme was up to 10 hours in the study by Johnson et al. [10] and 48 hours post-infusion done by Katz et al. [8] compared with a period of 24 hours in the present study. Additionally, in the study performed by Shibutani et al. investigating obese and lean adults [6], blood specimens were not obtained using a timed sampling scheme. Instead, samples remaining from arterial blood gas analyses were analyzed for fentanyl at median 24.4 and 24.6 hours after starting an infusion, respectively. The laboratory assay that was used for fentanyl quantification also accounts for inter-study variability. It is well known that cross-reactivity may occur with structurally related or matrix compounds when a radioimmunoassay is used, as it was the case in the studies of Johnson et al. [10], Katz et al. [8], and Shibutani et al. [6]. In the present study a LC-MS/MS assay unambiguously identifying fentanyl was used [11].
While 39 obese and 70 lean patients participated in the clinical trial of Shibutani et al. [6], only pharmacokinetic data of 10 obese and 16 lean patients were available who received fentanyl infusions at constant doses also in the postoperative period. In addition, steady state was assumed when clearance was calculated. While the obese adults were significantly older than our study population, no significant difference was observed in total body weight (117.3 ± 33.4 kg vs. 137.4 ± 14.3, p=0.12) and fentanyl clearance calculated normalized to TBW (8.76 ± 1.74 mL/min*kg vs. 11.2 ± 2.6 mL/min*kg, p=0.08), but in absolute fentanyl clearance (986 ± 155 mL/min vs. 1522 ± 310, p=0.006). The calculated absolute fentanyl clearance was lower in lean adults (718 ± 163 mL/min) than in obese adults with the sampling time point always being more than 12 hours since start of the continuous infusion [6].
When the data of Shibutani et al. [6] are compared to studies in healthy adults, the absolute clearance values of the lean adults (mean body weight: 68.5 ± 9.5 kg) and the obese adults are lower than previously published data by Ibrahim et al. (15.3 ± 5.0 mL/min/kg, calculated for the mean body weight of 74 kg: 1132 mL/min [16]), McClain et al. (11.6 ± 2.6 mL/min/kg and 882.1 ± 187.1 mL/min [4]), Olkkola et al. (15.6 ± 8.2 mL/min/kg, calculated for 70 kg body weight: 1092 mL/min [17]), Palkama et al. (23.9 ± 9.9 mL/min/kg, calculated for 70 kg body weight: 1673 mL/min [18]), Saari et al. (14.0 ± 2.5 mL/min/kg, calculated for 70 kg body weight: 980 mL/min [19]) and Ziesenitz et al. (19.0 ± 6.8 mL/min/kg and 1388 ± 487 mL/min [20], all data given as mean ± SD, if available). With the exception of the study by Palkama et al., if these data are compared with the present study (11.2 ± 2.6 mL/min/kg and 1522 ± 310 mL/min), the clearance of the adolescents with clinically severe obesity lies above the described ranges, when using absolute values (mL/min) for the total body clearance.
The values of the volume of distribution of fentanyl, for which no data were available in the study published by Shibutani et al., in the present study (4.7 ± 2.1 L/kg and 635 ± 282 L) are in the upper range of published values if compared to data by Ibrahim et al. 7.2 ± 2.4 L/kg, calculated for the mean body weight of 74 kg: 533 L [16]), McClain et al. (4.1 ± 0.4 L/kg and 311.0 ± 39.1 L [4]), Olkkola et al. (9.3 ± 4.9 L/kg, calculated for 70 kg body weight: 651 L [17]), Palkama et al. (5.2 ± 1.9 L/kg, calculated for 70 kg body weight: 364 L [18]), Saari et al. (9.5 ± 2.4 L/kg, calculated for 70 kg body weight: 665 L [19]) and Ziesenitz et al. (11.5 ± 6.6 L/kg and 832 ± 452 L [20], all data given as mean ± SD, if available). This might be due to the higher body fat mass in the adolescents with clinically severe obesity.
4.2 Factors influencing fentanyl PK
It has been claimed that fentanyl is mainly metabolized to norfentanyl by CYP3A [1]. Fentanyl, however, is a high extraction-ratio drug whose metabolism is rather dependent on hepatic blood flow than on enzymatic capacity [3]. Therefore, CYP3A4 seems not to be the major determinant of fentanyl total body clearance [20].
Non-alcoholic fatty liver disease (NAFLD) may alter the expression of specific drug metabolizing enzymes and drug transporters [21], which could influence the pharmacokinetics of drugs metabolized by the liver. The effect of fatty infiltration of the liver on pharmacokinetics is difficult to quantify since there can be large variations in liver texture and individual enzymatic performance [15]. While NAFLD results in increased fat deposition in the liver, leading to narrowing of the sinusoids and altering the liver morphology which may impact hepatic blood flow [22], the latter does not necessarily need to be reduced since cardiac output as well as circulating blood volume, and thus, splanchnic blood flow, are increased in obese patients [3, 23]. Thus, a clear correlation between liver disease described by standard liver function tests, such alanine aminotransferase (ALAT) or aspartate aminotransferase (ASAT), could not be established up to date [15]. In the present study, however, an alteration of PK by NAFLD seemed unlikely, especially because NAFLD is less often seen in obese adolescents compared to obese adults [24]. We therefore attribute the increased fentanyl clearance observed in our patient cohort to an increased hepatic blood flow as suggested previously.
4.3 Comparison of weight descriptors
Our current practice of dosing fentanyl based on IBW was adopted from standard adult bariatric anesthesia procedures since there are no peer-reviewed perioperative guidelines but only recommendations for fentanyl use in obese children and adolescents [25]. As dosing on TBW would overestimate the obese patients’ fentanyl requirements with the potential for overdosing [26], it was implemented to dose fentanyl based on IBW. Several authors suggested to titrate fentanyl dose according to clinical effect after initial LBM-based dosing [5, 26, 27] while Mulla et al., and Adams and Murphy suggested to use a loading dose based on TBW and a maintenance dose based on LBW (or even body surface area) which had also been recommended for sufentanil [15, 28]. While this approach can be justified from a pharmacokinetic point of view based on the current study due to a similar volume of distribution per kg bodyweight and an increased clearance per kg bodyweight, the PD of fentanyl and thus the potential adverse effect of respiratory depression should not be omitted. The dosing scalar “pharmacokinetic mass” had been introduced by Shibutani et al. [6] for fentanyl dosing in obese adults since fentanyl clearance seems to increase non-linearly with increasing body weight [29]. A recent review on anesthetic considerations for adolescent bariatric surgery recommended using LBW as dosing weight for fentanyl, up to a maximum of 100 kg LBW [30].
4.4 Limitations of the study
A limitation of the study is the small sample size, but due to the fact that no data on fentanyl PK was available in this particular patient group, we first aimed at conducting an exploratory pilot study.
Possible variations in fentanyl PK when compared to other studies may be caused by drug-drug interactions, since the patients received multiple medications. There were, however, no significant pharmacokinetic drug interactions documented in the summary of product characteristics between the medications taken by the study participants and fentanyl.
Differences in fentanyl clearance or volume of distribution between adolescents with clinically severe obesity and control groups of lean children [10] as well as obese and lean adults have been observed [6] which may neither be explained by the weight difference nor by different study designs alone. Using historical control data as comparison may introduce confounding errors. These errors may result from different study settings, such as the surgical or intensive care units, changes in the state of the art techniques for general anesthesia, like the use of new drug combinations, and perioperative pain management, as well as differences in the analytical methods used, assay sensitivity, and the methods of PK analysis.
Conclusion
This is the first study which focuses on the pharmacokinetics of fentanyl in adolescent patients with clinically severe obesity undergoing bariatric surgery. Compared with previously obtained data, our results suggest that fentanyl clearance in obese adolescents with clinically severe obesity is enhanced compared to sparse previously published data. While the results of the current study yield that the loading dose of fentanyl may be based on TBW followed by maintenance doses based on LBW and IBW, we want to emphasize that clinically severely obese patients are more at risk for respiratory side effects of fentanyl and the pharmacodynamics should also be taken into consideration when dosing fentanyl. Based on the present results, we suggest that our current practice of bolus dosing fentanyl based upon IBW during adolescent bariatric anesthesia may be appropriate in the absence of definitive pharmacodynamic assessments.
Further investigation is required including pharmacodynamic characterization to determine appropriate dosing of fentanyl in this unique patient population.
Figure 1.
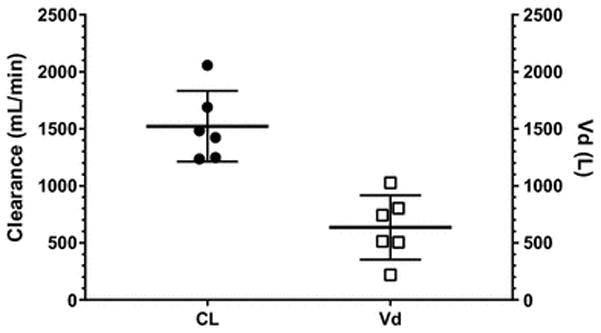
Fentanyl total body clearance (full circles)and volume of distribution (open squares) in adolescents with clinically severe obesity.
Key Points.
Despite an increasing number of obese pediatric patients undergoing surgery, there are no pharmacokinetic data available for dosing recommendations of fentanyl in this unique patient population.
Clinically severe obese adolescents seem to demonstrate an increase in fentanyl clearance compared to their lean counterparts.
Acknowledgments
This project was supported by Award Number UL1RR031988 from the NIH National Center for Research Resources. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Research Resources or the NIH.
Footnotes
Study registration: NCT01955993 (clinicaltrials.gov)
Compliance with Ethical Standards
Disclosure of potential conflicts of interest:
This project was supported by Award Number UL1RR031988 from the NIH National Center for Research Resources. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Research Resources or the NIH.
All authors (JDV, VCZ, EFW, AM, RB, GS, JW, GM, JNvdA) declare that they have no conflict of interest.
Research involving Human Participants and/or Animals:
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent:
Informed consent was obtained from all individual participants included in the study.
References
- 1.Tateishi T, Krivoruk Y, Ueng YF, Wood AJ, Guengerich FP, Wood M. Identification of human liver cytochrome P-450 3A4 as the enzyme responsible for fentanyl and sufentanil N-dealkylation. Anesth Analg. 1996 Jan;82(1):167–72. doi: 10.1097/00000539-199601000-00031. [DOI] [PubMed] [Google Scholar]
- 2.Murphy MR, Hug CC, Jr, McClain DA. Dose-independent pharmacokinetics of fentanyl. Anesthesiology. 1983 Dec;59(6):537–40. doi: 10.1097/00000542-198312000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Brill MJ, Diepstraten J, van Rongen A, van Kralingen S, van den Anker JN, Knibbe CA. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet. 2012 May 1;51(5):277–304. doi: 10.2165/11599410-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.McClain DA, Hug CC., Jr Intravenous fentanyl kinetics. Clin Pharmacol Ther. 1980 Jul;28(1):106–14. doi: 10.1038/clpt.1980.138. [DOI] [PubMed] [Google Scholar]
- 5.Leykin Y, Miotto L, Pellis T. Pharmacokinetic considerations in the obese. Best Pract Res Clin Anaesthesiol. 2011 Mar;25(1):27–36. doi: 10.1016/j.bpa.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Shibutani K, Inchiosa MA, Jr, Sawada K, Bairamian M. Accuracy of pharmacokinetic models for predicting plasma fentanyl concentrations in lean and obese surgical patients: derivation of dosing weight (“pharmacokinetic mass”) Anesthesiology. 2004 Sep;101(3):603–13. doi: 10.1097/00000542-200409000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Hertzka RE, Gauntlett IS, Fisher DM, Spellman MJ. Fentanyl-induced ventilatory depression: effects of age. Anesthesiology. 1989 Feb;70(2):213–8. doi: 10.1097/00000542-198902000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Katz R, Kelly HW. Pharmacokinetics of continuous infusions of fentanyl in critically ill children. Crit Care Med. 1993 Jul;21(7):995–1000. doi: 10.1097/00003246-199307000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Singleton MA, Rosen JI, Fisher DM. Plasma concentrations of fentanyl in infants, children and adults. Can J Anaesth. 1987 Mar;34(2):152–5. doi: 10.1007/BF03015333. [DOI] [PubMed] [Google Scholar]
- 10.Johnson KL, Erickson JP, Holley FO, Scott JC. Fentanyl pharmacokinetics in the pediatric population. Anesthesiology. 1984;61(3A):1. [Google Scholar]
- 11.Mahlke NS, Ziesenitz V, Mikus G, Skopp G. Quantitative low-volume assay for simultaneous determination of fentanyl, norfentanyl, and minor metabolites in human plasma and urine by liquid chromatography-tandem mass spectrometry (LC-MS/MS) Int J Legal Med. 2014 Sep;128(5):771–8. doi: 10.1007/s00414-014-1040-y. [DOI] [PubMed] [Google Scholar]
- 12.Freid EB, Miles MV, Nocera MA, Zaritsky AL. Prolonged Continuous Infusions of Fentanyl or Alfentanil in Critically Ill Children - Pharmacokinetics and Pharmacodynamics. Anesthesiology. 1994 Sep;81(3A):A257-A. [Google Scholar]
- 13.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA : the journal of the American Medical Association. 2014 Feb 26;311(8):806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams JP, Murphy PG. Obesity in anaesthesia and intensive care. Br J Anaesth. 2000 Jul;85(1):91–108. doi: 10.1093/bja/85.1.91. [DOI] [PubMed] [Google Scholar]
- 15.Cheymol G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet. 2000 Sep;39(3):215–31. doi: 10.2165/00003088-200039030-00004. [DOI] [PubMed] [Google Scholar]
- 16.Ibrahim AE, Feldman J, Karim A, Kharasch ED. Simultaneous assessment of drug interactions with low- and high-extraction opioids: application to parecoxib effects on the pharmacokinetics and pharmacodynamics of fentanyl and alfentanil. Anesthesiology. 2003 Apr;98(4):853–61. doi: 10.1097/00000542-200304000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Olkkola KT, Palkama VJ, Neuvonen PJ. Ritonavir’s role in reducing fentanyl clearance and prolonging its half-life. Anesthesiology. 1999 Sep;91(3):681–5. doi: 10.1097/00000542-199909000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Palkama VJ, Neuvonen PJ, Olkkola KT. The CYP 3A4 inhibitor itraconazole has no effect on the pharmacokinetics of i.v. fentanyl. Br J Anaesth. 1998 Oct;81(4):598–600. doi: 10.1093/bja/81.4.598. [DOI] [PubMed] [Google Scholar]
- 19.Saari TI, Laine K, Neuvonen M, Neuvonen PJ, Olkkola KT. Effect of voriconazole and fluconazole on the pharmacokinetics of intravenous fentanyl. Eur J Clin Pharmacol. 2008 Jan;64(1):25–30. doi: 10.1007/s00228-007-0398-x. [DOI] [PubMed] [Google Scholar]
- 20.Ziesenitz VC, Konig SK, Mahlke NS, Skopp G, Haefeli WE, Mikus G. Pharmacokinetic interaction of intravenous fentanyl with ketoconazole. J Clin Pharmacol. 2015 Jun;55(6):708–17. doi: 10.1002/jcph.469. [DOI] [PubMed] [Google Scholar]
- 21.Woolsey SJ, Mansell SE, Kim RB, Tirona RG, Beaton MD. CYP3A Activity and Expression in Nonalcoholic Fatty Liver Disease. Drug Metab Dispos. 2015 Oct;43(10):1484–90. doi: 10.1124/dmd.115.065979. [DOI] [PubMed] [Google Scholar]
- 22.Casati A, Putzu M. Anesthesia in the obese patient: pharmacokinetic considerations. J Clin Anesth. 2005 Mar;17(2):134–45. doi: 10.1016/j.jclinane.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Farrell GC, Teoh NC, McCuskey RS. Hepatic microcirculation in fatty liver disease. Anatomical record. 2008 Jun;291(6):684–92. doi: 10.1002/ar.20715. [DOI] [PubMed] [Google Scholar]
- 24.Schwimmer JB. Clinical advances in pediatric nonalcoholic fatty liver disease. Hepatology. 2016 May;63(5):1718–25. doi: 10.1002/hep.28441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross PA, Scott GM. Childhood obesity: a growing problem for the pediatric anesthesiologist. Seminars in Anesthesia, Perioperative Medicine and Pain. 2006;25(3):142–8. [Google Scholar]
- 26.Schumann R. Anaesthesia for bariatric surgery. Best Pract Res Clin Anaesthesiol. 2011 Mar;25(1):83–93. doi: 10.1016/j.bpa.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Ingrande J, Lemmens HJ. Dose adjustment of anaesthetics in the morbidly obese. Br J Anaesth. 2010 Dec;105( Suppl 1):i16–23. doi: 10.1093/bja/aeq312. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz AE, Matteo RS, Ornstein E, Young WL, Myers KJ. Pharmacokinetics of sufentanil in obese patients. Anesth Analg. 1991 Dec;73(6):790–3. [PubMed] [Google Scholar]
- 29.Shibutani K, Inchiosa MA, Jr, Sawada K, Bairamian M. Pharmacokinetic mass of fentanyl for postoperative analgesia in lean and obese patients. Br J Anaesth. 2005 Sep;95(3):377–83. doi: 10.1093/bja/aei195. [DOI] [PubMed] [Google Scholar]
- 30.Samuels PJ, Sjoblom MD. Anesthetic considerations for pediatric obesity and adolescent bariatric surgery. Current opinion in anaesthesiology. 2016 Jun;29(3):327–36. doi: 10.1097/ACO.0000000000000330. [DOI] [PubMed] [Google Scholar]


