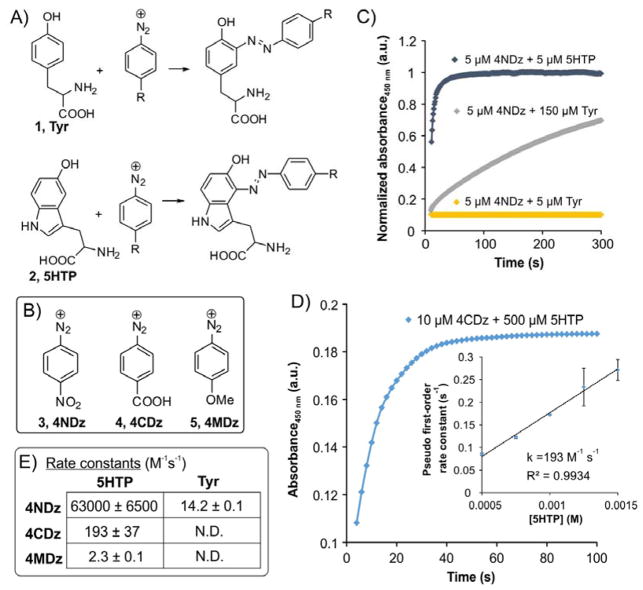
Figure 1.
5HTP 2 exhibit significantly higher reactivity toward aryl diazonium ions relative to Tyr 1. (A) Azo-coupling reaction of aryldiazoniums with Tyr and 5HTP. (B) Structures of aryl-diazonium ions used. (C) Observed rate of azo-coupling reaction of 4NDz 3 with 5HTP and Tyr at indicated concentrations in 100 mM phosphate buffer (pH 7, room temperature). (D) Measurement of the rate of the azo-coupling reaction between 4CDz and 5HTP under pseudo-first-order conditions. (E) Second-order rate constants of the azo-coupling reactions between the indicated partners (M−1 s −1). Each rate represents an average of three independent experiments, and error represents standard deviation.