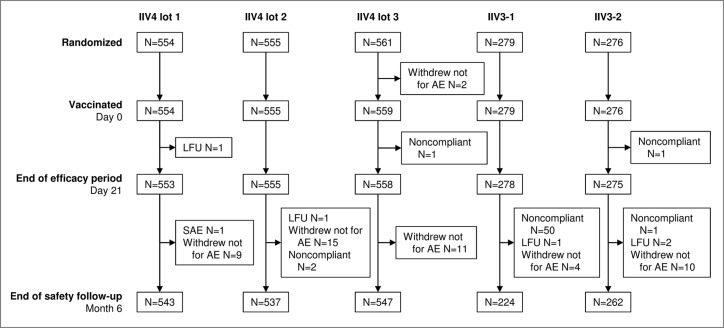
Figure 1.
Disposition of participants in the study. 2225 participants were included and randomized 2:2:2:1:1 to receive a single dose of one of the three lots of the 2014–2015 formulation of IIV4, IIV3-1, or IIV3-2. IIV4 contained the A(H1N1), A(H3N2), B Victoria lineage, and B Yamagata lineage strains; IIV3-1 contained the two A strains and the B Victoria lineage strain (IIV3-1); IIV3-2 was the 2014–2015 licensed IIV3 and contained the two A strains and the B Yamagata lineage strain. All but three participants were vaccinated. Reasons for discontinuation included a severe adverse event (SAE), voluntary withdrawal not for an adverse event (AE), noncompliance with the study procedures, and loss to follow-up (LFU). The high number of protocol violations in the IIV3-1 group after day 21 and before month 6 was due to 47 participants who were offered and accepted to receive a second vaccination with the commercial vaccine (IIV3-2) so that they would be covered for the B/Yamagata-lineage strain.