Abstract
Aim of the study
Probiotic administration to preterm infants has the potential to prevent necrotising enterocolitis (NEC). Data from randomised controlled trials (RCT) are conflicting but meta-analyses seem to support this intervention. To date, these analyses have not focused on surgical NEC. We aimed to determine the effect of probiotic administration to preterm infants on prevention of surgical NEC.
Methods
A systematic review of RCTs of probiotic administration to preterm infants was performed. Studies were included if RCT outcomes included any of (1) Bell’s stage 3 NEC; (2) surgery for NEC; and (3) deaths attributable to NEC. Article selection and data extraction were performed independently by two authors; conflicts were adjudicated by a third author. Data were meta-analysed using Review Manager V.5.3. A random effects model was decided on a priori because of the heterogeneity of study design; data are risk ratio (RR) with 95% CI.
Main results
Thirty-five RCTs reported NEC as an outcome. Seventeen reported surgical NEC; all RCTs were included. A variety of probiotic products was administered across studies. Description of surgical NEC in most studies was poor. Only 6/16 specifically reported incidence of surgery for NEC, 12/17 Bell’s stage 3 and 13/17 NEC-associated mortality. Although there was a trend towards probiotic administration reducing stage 3 NEC, this was not significant (RR 0.74 (0.52–1.05), p=0.09). There was no effect of probiotics on the RR of surgery for NEC (RR 0.84 (0.56–1.25), p=0.38). Probiotics did, however, reduce the risk of NEC-associated mortality (RR 0.56 (0.34–0.93), p=0.03).
Conclusion
Despite 35 RCTs on probiotic prevention of NEC, evidence for prevention of surgical NEC is not strong, partly due to poor reporting. In studies included in this meta-analysis, probiotic administration was associated with a reduction in NEC-related mortality.
What is already known on this topic?
In various randomised controlled trials (RCT) and meta-analyses, it was suggested that probiotic administration is associated with decrease in incidence of definite necrotising enterocolitis (NEC).
The evidence that probiotic administration is associated with a decreased incidence of surgical NEC is limited.
This is due to poor reporting of surgical NEC in RCTs and we urge better reporting of surgical aspects of NEC in future trials.
What this study hopes to add?
The reporting of surgical aspects of NEC in RCTs of probiotic administration is poor.
Probiotic administration is associated with a decrease in NEC-associated mortality.
Introduction
Although necrotising enterocolitis (NEC) is the most common life-threatening surgical emergency affecting neonates, we still do not know how to prevent or medically treat the disease.1 Many infants with NEC may have surgery with the aim of removal of necrotic intestine. Although the indications for surgery are not well defined, radiological evidence for intestinal perforation is often regarded as an absolute indication for surgery, and many surgeons would operate for failure to improve, or clinical deterioration, in response to medical management such as cessation of enteral feeds, antibiotic treatment and supportive treatment.2 In the last few years, there has been a surge of interest in the potential role of probiotics to prevent NEC and this has resulted in the publication of many randomised controlled trials (RCT), followed by systematic reviews and meta-analyses of these RCTs.3–6 Some commentators have asserted that it is ‘almost unethical’ to withhold probiotic administration to all preterm infants in order to prevent NEC.6 As the type/strain, dose, duration and timing of probiotics are not standardised, others find the evidence less compelling.7 The American Pediatric Surgical Association Outcomes and Clinical Trials Committee8 considered the level of evidence for routine probiotic supplementation and concluded that available data supported the routine supplementation of premature infants with probiotics although no conclusions could be drawn for the extremely low birth weight population (ie, those with the highest incidence of NEC) due to lack of data. However, most RCTs, and the systematic reviews and meta-analyses that result, focus on the development of confirmed NEC (ie, Bell’s stage 2) and not on the potential effect of probiotic administration on surgical NEC. We focused on surgical NEC since there is general recognition that infants who are treated surgically have more advanced disease than those who are managed medically and importantly are noted to have worse outcomes including higher mortality, more frequent need for further surgery and greater long-term neurodevelopmental impairment. The aim of this study was to perform a systematic review and meta-analysis in order to compare the effects of probiotic administration and placebo on surgical NEC in preterm infants.
Methods
A systematic review of available literature (Ovid Medline January 1974–June 2017) was conducted using the search strategy (probiotic* OR pro-biotic* or probio* OR lactobacill* OR bifidobacter* OR saccharomyces* OR bacillus) AND ((necrotizing enterocolitis or necrotising enterocolitis or necrotizing entero-colitis or necrotising entero-colitis) OR (necrot* and (enterocoli* or entero-coli*)) OR (‘necrotizing’ or ‘entero-colitis’ or ‘enterocolitis’) and NEC) AND publication type randomized controlled trial. A similar search was also conducted in Ovid Embase (January 1980–June 2017). Hand searching of the reference lists of published studies and citation searching using Web of Knowledge (Thomson-Reuter) were also performed in order to identify additional studies. A formal protocol was not prepared for this study.
Inclusion criteria were defined as follows: (1) RCT; (2) study compared enteral probiotics to placebo or no treatment; (3) study population defined as premature infants; (4) explicit data available on incidence of either (A) Bell’s stage 3 NEC, (B) surgery for NEC or (C) NEC-associated mortality. Initial screening for inclusion was performed independently by two authors using the online tool Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia, available at http://www.covidence.org). Adjudication regarding inclusion/exclusion was performed by the other two authors. The following data were extracted: number of infants treated with probiotic/placebo, infants with/without Bell’s stage 3 NEC, infants having surgery for NEC (including peritoneal drain), Bell’s stage 2/3 NEC, deaths attributable to NEC. Data on Bell’s stage 2/3 NEC, the outcome measure most frequently reported in meta-analyses of probiotics for prevention of NEC, were extracted from included papers (ie, only those including surgical outcomes) in order to determine whether this subset of papers was representative of the larger group of studies with broader inclusion criteria.
Data were meta-analysed using Review Manager V.5.3. A random effects model was decided on a priori because of the heterogeneity of study design; data are risk ratio (RR) with 95% CI; heterogeneity was assessed using I2 and associated χ2 test, and funnel plots prepared for assessment of bias across studies. An additional analysis (not preplanned) using bacterial products only was also performed. Power calculations were performed using an online tool (Sealed Envelope 2012), power calculator for binary outcome superiority trial, available at: https://www.sealedenvelope.com/power/binary-superiority/ using an α error of 5% and β of 80%.
Results
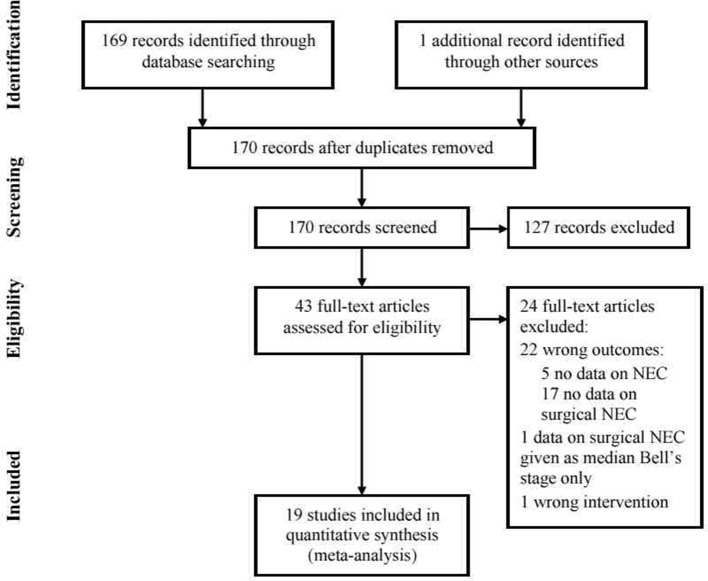
The search strategy yielded 169 abstracts, and further searching an additional abstract that was potentially eligible. Full-text screening as described in the Methods section led to the selection of 19 articles for inclusion9–27 and 24 articles for exclusion.28–51 A flow chart indicating screening, inclusion and exclusion of studies is shown in figure 1. Characteristics of the 19 included studies are shown in table 1. The lack of consistency and clarity regarding definition of NEC as an RCT outcome, and reporting of surgical NEC (Bell’s stage 3, infants having surgery for NEC) were notable. Most excluded papers did not report surgical NEC; two further papers26 27 were included as it was possible to extract data on surgical outcomes from the papers only because there was a zero incidence of NEC in either arm (and therefore a zero incidence of surgical NEC) rather than explicit reporting of surgical NEC in the RCT outcomes.
Figure 1.
Flow chart showing selection of studies for inclusion in the systematic review and meta-analysis. NEC, necrotising enterocolitis.
Table 1.
Characteristics of included studies
| Study | Year | Probiotic used | Placebo | Number of patients | Primary outcome defined | Bell’s stage 3 | Surgery for NEC | Death from NEC | ||
| Probiotic | Placebo | |||||||||
| Al-Hosni et al10 | 2012 | Lactobacillus rhamnosus GG+Bifidobacterium infantis in milk | Unsupplemented milk | 50 | 51 | Weight | 501–1000 g | x | x | |
| Bin-Nun et al11 | 2005 |
Lactobacillus bifidus, Streptococcus thermophilus
and B. infantis in milk |
Unsupplemented milk | 72 | 73 | NEC | <1500 g BW | x | x | |
| Costeloe et al12 | 2015 | Bifidobacterium breve BBG-001 in milk | Unsupplemented formula | 654 | 661 | NEC (stage 2 or 3) | 23–30 weeks GA | x | x | x |
| Dani et al13 | 2002 | Lactobacillus GG in milk | Maltodextrins in milk | 295 | 290 | Urinary tract infection, bacterial sepsis and NEC | <33 weeks GA or <1500 g BW | x | x | |
| Demirel et al14 | 2013 | Saccharomyces boulardii | No addition | 135 | 136 | NEC stage ≥2, death | <32 weeks GA and <1500 g BW | x | x | |
| Fernández-Carrocera et al9 | 2013 |
Lactobacillus 4 spp Bifidobacterium infantis S. thermophilus |
No addition | 75 | 75 | NEC | <1500 g BW | x | x | |
| Jacobs et al15 | 2013 | B. infantis, S. thermophilus and Bifidobacterium lactis | Maltodextrin | 548 | 551 | Late-onset sepsis | <32 weeks GA and BW <1500 g | x | ||
| Lin et al16 | 2005 | Lactobacillus acidophilus and B. infantis | No addition | 180 | 187 | NEC, death, sepsis | <1500 g BW | x | x | |
| Lin et al17 | 2008 | L. acidophilus and Bifidobacterium bifidum | No addition | 217 | 217 | NEC, death | <1500 g | x | x | |
| Manzoni et al18 | 2006 | Lactobacillus casei sp rhamnosus | No addition | 39 | 41 | Enteric fungal colonisation | <1500 g | x | x | x |
| Oncel et al19 | 2014 | Lactobacillus reuteri in oil base | Oil base | 200 | 200 | Death, NEC | <32 weeks GA and <1500 g BW | x | ||
| Rougé et al20 | 2009 | Bifidobacterium longum and L. rhamnosus | Maltodextrin | 45 | 49 | Enteral feeding | <32 weeks GA and <1500 g BW | x | ||
| Saengtawesin et al21 | 2014 | L. acidophilus and B. bifidum | No addition | 31 | 29 | NEC, death | <34 weeks GA and <1500 g BW | x | x | |
| Sari et al22 | 2011 | Lactobacillus sporogenes | No addition | 110 | 111 | NEC, death | <33 weeks GA and <1500 g BW | x | x | x |
| Serce et al23 | 2013 | S. boulardii | Distilled water | 104 | 104 | NEC, sepsis, death | <34 weeks GA and <1500 g BW | x | ||
| Tewari et al24 | 2015 | Bacillus clausii | Sterile water | 123 | 121 | Sepsis | <34 weeks GA | x | ||
| Totsu et al27* | 2014 | B. bifidum | Dextrin | 153 | 130 | Full enteral feeding | <1500 g | x | x | x |
| Van Niekerk et al25 | 2015 | L. rhamnosus and B. infantis | Medium chain triglyceride oil | 91 | 93 | NEC, sepsis | >500 g and <1250 g, breast milk fed | x | x | |
| Xu et al26* | 2016 | S. boulardii | No addition | 63 | 62 | Growth | 30–37 weeks and 1500–2500 g BW | x | x | x |
*Totsu et al and Xu et al both reported zero cases of NEC so other NEC outcomes are by definition zero.
BW, birth weight; GA, gestational age; NEC, necrotising enterocolitis.
Bell’s stage 2/3 NEC
Data on Bell’s stage 2/3 NEC were obtainable from 19/19 included studies9–27; incidence in the placebo group varied between 16% and 0%. Probiotic administration was associated with a significant reduction in the incidence of Bell’s stage 2/3 NEC (RR 0.64 (0.48, 0.84), p<0.002). There was a low degree of heterogeneity between studies (I2=14%, p=0.29).
Bell’s stage 3 NEC
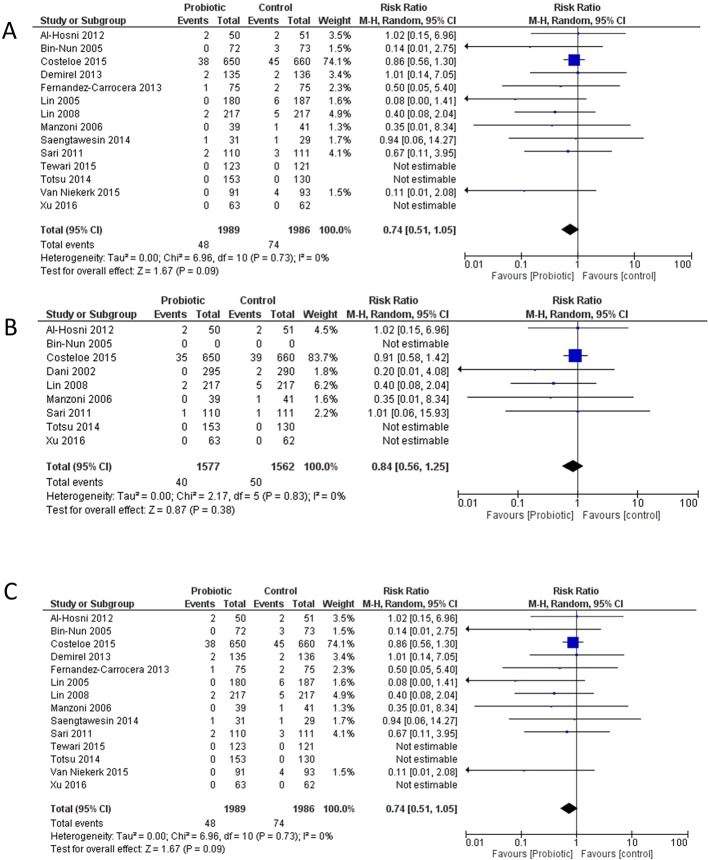
Data on Bell’s stage 3 as an outcome were available from 14/19 included studies,9–12 14 16–18 21 22 24–27 of which 12/19 explicitly reported Bell’s stage 39–12 14 16–18 21 22 24 25; incidence in the placebo group varied between 7% and 0%. Probiotic usage was not associated with a significant effect on the incidence of stage 3 NEC, although there was a trend towards a decrease, with a similar RR to that of Bell’s stage 2–3 NEC (RR 0.74 (0.51–1.05), p=0.09, figure 2). There was no evidence for significant heterogeneity (I2=0%, p=0.73).
Figure 2.
Meta-analysis of included studies. (A) Bell’s stage 3 NEC (necrotising enterocolitis) in infants who received probiotic or placebo. (B) Surgery for NEC in infants who received probiotic or placebo. (C) Mortality attributed to NEC in infants who received probiotic or placebo. df, degrees of freedom.
Surgery for NEC
Data on surgery for NEC were available from only 8/19 studies10 12 13 17 18 22 26 27 of which only 6/19 explicitly reported incidence of surgery for NEC.10 12 13 17 18 22 There was no effect of probiotics on the RR of surgery for NEC (RR 0.84 (0.56–1.25), p=0.38, figure 2). There was no evidence for significant heterogeneity (I2=0%, p=0.83).
Deaths attributable to NEC
Data on death attributable to NEC were available from 15/19 studies9 11–15 17 19–23 25–27; of these 13/19 explicitly reported death attributable to NEC.9 11–15 17 19–23 25 In one study, some deaths were reported as being from Bell’s stage 1; however, by consensus these deaths were not included as the diagnosis of NEC had not been confirmed.25 Probiotic administration was associated with a significant reduction in the risk of NEC-associated mortality (figure 2, RR 0.56 (0.34–0.93), p=0.03) with no evidence for significant heterogeneity (I2=0%, p=0.85).
Analysis by probiotic product
We repeated the analyses, excluding those using only a fungal probiotic product.14 23 26 Administration of bacterial probiotic products was associated with a significant reduction in the incidence of Bell’s stage 2/3 NEC (RR 0.57 (0.41, 0.80), p=0.001), a trend towards a decrease in Bell’s stage 3 NEC (RR 0.73 (0.50–1.05), p=0.09), no effect on the RR of surgery for NEC (RR 0.84 (0.56–1.25), p=0.38) and a significant reduction in the risk of NEC-associated mortality (RR 0.53 (0.31–0.90), p=0.02).
Assessment of bias
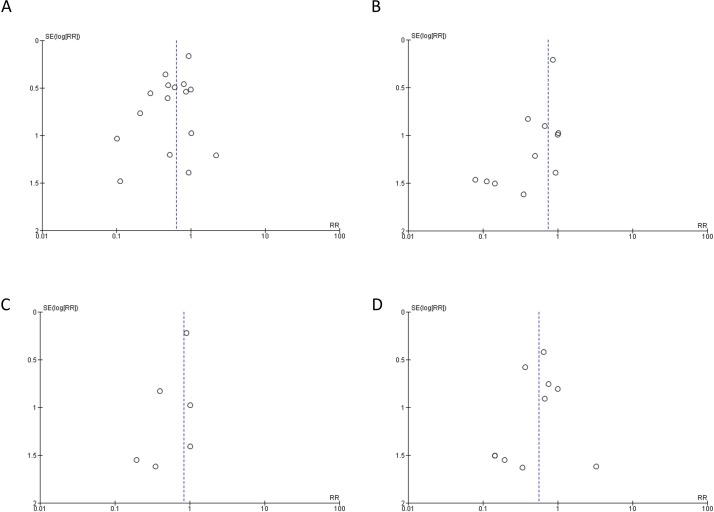
The risk of bias in individual studies is shown in online supplementary table 1. In order to assess evidence for publication bias, funnel plots for each of the outcomes (Bell’s stage 2/3, Bell’s stage 3, surgery for NEC and mortality attributable to NEC) were generated (figure 3). For each outcome, the apex of the funnel plot is an RR of <1, providing some limited evidence for bias towards publication of studies favouring probiotic administration, although the ability to detect publication bias is limited by the low number of publications for some of these outcomes.
Figure 3.
Funnel plots of included studies. (A) Bell’s stage 2–3 NEC in infants who received probiotic or placebo. (B) Bell’s stage 3 NEC in infants who received probiotic or placebo. (C) Surgery for NEC in infants who received probiotic or placebo. (D) Mortality attributed to NEC in infants who received probiotic or placebo. RR, risk ratio.
bmjpo-2017-000066supp001.docx (16.4KB, docx)
Discussion
Over the past 10 years, there have been many meta-analyses and Cochrane Reviews evaluating probiotic administration for the prevention of NEC.3–6 To our knowledge, none to date has specifically focused on whether or not probiotics reduce NEC requiring surgery. In our present systematic review, we found that surgical aspects of NEC were rather poorly reported in RCTs of probiotic administration; of 37 papers reporting any data on NEC as an RCT outcome, only 18 (49%) specifically reported surgical NEC (and in a further two data could be extrapolated due to the zero incidence of any NEC), and in one of these, data could not be used due to unconventional reporting of Bell’s staging.51
The available data from included papers suggest that probiotic administration was not associated with a significant decrease in the risk of developing Bell’s stage 3 NEC or having surgery for this condition. Previous meta-analyses of probiotics have shown a significant effect of probiotic administration in decreasing the incidence of Bell’s stage 2–3 NEC. In order to determine whether the 16 papers that specifically reported surgical NEC were representative of the larger group of papers that report Bell’s stage 2–3 NEC, we also analysed the effect of probiotic administration on Bell’s stage 2–3 NEC in the 16 papers reporting surgical NEC. Consistent with the findings of other meta-analyses with less restrictive inclusion criteria,3–5 52 we also demonstrated a significant decrease in the risk of developing Bell’s stage 2–3 NEC with probiotic administration. Although the risk of developing Bell’s stage 3 NEC was similar to that of developing stage 2–3 NEC, the difference in risk of Bell’s stage 3 disease was non-significant. This was due to wider CIs associated with a smaller number of patients.
This review has demonstrated a statistically significant effect of probiotics in reducing mortality attributed to NEC, with a relative risk of 0.56. It is of interest to analyse specifically mortality attributable to NEC as most meta-analyses examine all-cause mortality3–5 52 and we are not aware of any that have analysed mortality attributable to NEC. It may seem counterintuitive that probiotics significantly decrease the risk of NEC-associated mortality without a significant effect on the risk of surgical NEC. This can be explained first because more studies reported deaths than reported either Bell’s stage 3 or surgery for NEC. Second, up to 20% of infants who have been diagnosed as having definite NEC die without ever having an operation or a postmortem examination,53 and in addition, many of the studies reporting mortality from NEC did not have mortality as a defined primary or secondary endpoint.
There are a number of potentially confounding factors that should be considered when interpreting these results. None of the RCTs reviewed for this study included a protocol for the decision to proceed to surgery nor precise indications for surgery in infants with NEC. This is an important factor to consider given the decision or indication to perform surgery may differ between surgeons and centre.2 A further confounding issue is the likely inclusion of infants with spontaneous intestinal perforation (SIP) in reports of infants with NEC. Many surgeons have debated whether SIP and NEC are a similar disease but there is now greater acceptance that they are distinct disease entities. We are not aware of any reports that suggest probiotics influence the risk of developing SIP. Although our present study does not show evidence that probiotics reduce surgical NEC, we acknowledge that in the absence of consistent reporting of both indications for surgery and definitions of NEC/SIP, we should be cautious when generalising our findings. Diagnosis of NEC, staging of the disease according to Bell’s criteria, is a problematic area, and both pneumatosis intestinalis (the main criterion used to define Bell’s stage 2 NEC) and pneumoperitoneum (the main criterion used to define Bell’s stage 3 NEC) have poor interobserver agreement—even between expert radiologists.54–56 It is also worth noting that not all probiotic RCTs had independent radiologists.
There are many difficulties in meta-analysing probiotic trials. Cross colonisation of the placebo group is one, with data from one RCT12 suggesting that up to 37% of placebo allocated participants were colonised with the probiotic intervention after 2 weeks. Inconsistent and limited data reporting trial outcomes by colonisation status precluded such analyses in our present study, though data from one large RCT suggest non-significant trends towards reduced NEC in babies successfully colonised with probiotics. Furthermore, probiotics work through a diverse range of mechanistic actions and not all probiotics act via the same mechanism.57 One of the controversies in using probiotics relates to the uncertainty of which probiotic will achieve optimum benefit. In this meta-analysis, a variety of different probiotic products were used. Even if we concluded that probiotics were effective in preventing surgical NEC, we would not be able to recommend a specific product, strain, concentration or even species. Too few studies are available to be able to be meaningfully analysed by the type of probiotic administered.
Given the observed data, in order to detect a significant difference in Bell’s stage 3 NEC, an RCT would need to recruit 2757 patients in each arm. This is likely prohibitive, so we may never have robust evidence to answer the question of whether probiotic administration prevents surgical NEC. However, recent advances in understanding the microbiological basis for the development of NEC58 provide some hope that appropriately targeted probiotic therapies could be effective in reducing the devastating effects of this disease. In conducting future RCTs we recommend that robust reporting of surgical NEC, SIP and any abdominal surgery (eg, indications for surgery, operation performed, surgical outcomes) will allow us to more clearly assess the benefits of probiotic interventions.
Acknowledgments
None
Footnotes
Funding: SE gratefully acknowledges support from Great Ormond Street Children’s Charity and the NIHR Great Ormond Street Biomedical Research Centre.
Disclaimer: The funders had no input into the systematic review.
Competing interests: SE has received consultancy fees from Fresenius-Kabi and a speakers honorarium from Danone.
References
- 1.Hall NJ, Eaton S, Pierro A. Necrotizing enterocolitis: prevention, treatment, and outcome. J Pediatr Surg 2013;48:2359–67. doi:10.1016/j.jpedsurg.2013.08.006 [DOI] [PubMed] [Google Scholar]
- 2.Rees CM, Hall NJ, Eaton S, et al. . Surgical strategies for necrotising enterocolitis: a survey of practice in the United Kingdom. Arch Dis Child Fetal Neonatal Ed 2005;90:F152–F155. doi:10.1136/adc.2004.051862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. The Cochrane database of systematic reviews 2014;4:CD005496. [DOI] [PubMed] [Google Scholar]
- 4.Lau CS, Chamberlain RS. Probiotic administration can prevent necrotizing enterocolitis in preterm infants: a meta-analysis. J Pediatr Surg 2015;50:1405–12. doi:10.1016/j.jpedsurg.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 5.Wang Q, Dong J, Zhu Y. Probiotic supplement reduces risk of necrotizing enterocolitis and mortality in preterm very low-birth-weight infants: an updated meta-analysis of 20 randomized, controlled trials. J Pediatr Surg 2012;47:241–8. doi:10.1016/j.jpedsurg.2011.09.064 [DOI] [PubMed] [Google Scholar]
- 6.Deshpande G, Rao S, Patole S, et al. . Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics 2010;125:921–30. doi:10.1542/peds.2009-1301 [DOI] [PubMed] [Google Scholar]
- 7.Mihatsch WA, Braegger CP, Decsi T, et al. . Critical systematic review of the level of evidence for routine use of probiotics for reduction of mortality and prevention of necrotizing enterocolitis and sepsis in preterm infants. Clin Nutr 2012;31:6–15. doi:10.1016/j.clnu.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 8.Downard CD, Renaud E, St Peter SD, et al. . Treatment of necrotizing enterocolitis: an American Pediatric Surgical Association Outcomes and Clinical Trials Committee systematic review. J Pediatr Surg 2012;47:2111–22. doi:10.1016/j.jpedsurg.2012.08.011 [DOI] [PubMed] [Google Scholar]
- 9.Fernández-Carrocera LA, Solis-Herrera A, Cabanillas-Ayón M, et al. . Double-blind, randomised clinical assay to evaluate the efficacy of probiotics in preterm newborns weighing less than 1500 g in the prevention of necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed 2013;98:F5–F9. doi:10.1136/archdischild-2011-300435 [DOI] [PubMed] [Google Scholar]
- 10.Al-Hosni M, Duenas M, Hawk M, et al. . Probiotics-supplemented feeding in extremely low-birth-weight infants. J Perinatol 2012;32:253–9. doi:10.1038/jp.2011.51 [DOI] [PubMed] [Google Scholar]
- 11.Bin-Nun A, Bromiker R, Wilschanski M, et al. . Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr 2005;147:192–6. doi:10.1016/j.jpeds.2005.03.054 [DOI] [PubMed] [Google Scholar]
- 12.Costeloe K, Hardy P, Juszczak E, et al. . Probiotics in Preterm Infants Study Collaborative Group. Bifidobacterium breve BBG-001 in very preterm infants: a randomised controlled phase 3 trial. Lancet 2016;387:649–60. doi:10.1016/S0140-6736(15)01027-2 [DOI] [PubMed] [Google Scholar]
- 13.Dani C, Biadaioli R, Bertini G, et al. . Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. A prospective double-blind study. Biol Neonate 2002;82:103–8. doi:10.1159/000063096 [DOI] [PubMed] [Google Scholar]
- 14.Demirel G, Erdeve O, Celik IH, et al. . Saccharomyces boulardii for prevention of necrotizing enterocolitis in preterm infants: a randomized, controlled study. Acta Paediatr 2013;102:e560–5. doi:10.1111/apa.12416 [DOI] [PubMed] [Google Scholar]
- 15.Jacobs SE, Tobin JM, Opie GF, et al. . Probiotic effects on late-onset sepsis in very preterm infants: a randomized controlled trial. Pediatrics 2013;132:1055–62. doi:10.1542/peds.2013-1339 [DOI] [PubMed] [Google Scholar]
- 16.Lin HC, Su BH, Chen AC, et al. . Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics 2005;115:1–4. doi:10.1542/peds.2004-1463 [DOI] [PubMed] [Google Scholar]
- 17.Lin HC, Hsu CH, Chen HL, et al. . Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics 2008;122:693–700. doi:10.1542/peds.2007-3007 [DOI] [PubMed] [Google Scholar]
- 18.Manzoni P, Mostert M, Leonessa ML, et al. . Oral supplementation with Lactobacillus casei subspecies rhamnosus prevents enteric colonization by Candida species in preterm neonates: a randomized study. Clin Infect Dis 2006;42:1735–42. doi:10.1086/504324 [DOI] [PubMed] [Google Scholar]
- 19.Oncel MY, Sari FN, Arayici S, et al. . Lactobacillus Reuteri for the prevention of necrotising enterocolitis in very low birthweight infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 2014;99:F110–5. doi:10.1136/archdischild-2013-304745 [DOI] [PubMed] [Google Scholar]
- 20.Rougé C, Piloquet H, Butel MJ, et al. . Oral supplementation with probiotics in very-low-birth-weight preterm infants: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr 2009;89:1828–35. doi:10.3945/ajcn.2008.26919 [DOI] [PubMed] [Google Scholar]
- 21.Saengtawesin V, Tangpolkaiwalsak R, Kanjanapattankul W. Effect of oral probiotics supplementation in the prevention of necrotizing enterocolitis among very low birth weight preterm infants. J Med Assoc Thai 2014;97(Suppl 6):S20–5. [PubMed] [Google Scholar]
- 22.Sari FN, Dizdar EA, Oguz S, et al. . Oral probiotics: Lactobacillus sporogenes for prevention of necrotizing enterocolitis in very low-birth weight infants: a randomized, controlled trial. Eur J Clin Nutr 2011;65:434–9. doi:10.1038/ejcn.2010.278 [DOI] [PubMed] [Google Scholar]
- 23.Serce O, Benzer D, Gursoy T, et al. . Efficacy of Saccharomyces boulardii on necrotizing enterocolitis or sepsis in very low birth weight infants: a randomised controlled trial. Early Hum Dev 2013;89:1033–6. doi:10.1016/j.earlhumdev.2013.08.013 [DOI] [PubMed] [Google Scholar]
- 24.Tewari VV, Dubey SK, Gupta G. Bacillus clausii for prevention of late-onset sepsis in preterm infants: a randomized controlled trial. J Trop Pediatr 2015;61:377–85. doi:10.1093/tropej/fmv050 [DOI] [PubMed] [Google Scholar]
- 25.Van Niekerk E, Nel DG, Blaauw R, et al. . Probiotics reduce necrotizing enterocolitis severity in HIV-exposed premature infants. J Trop Pediatr 2015;61:155–64. doi:10.1093/tropej/fmv004 [DOI] [PubMed] [Google Scholar]
- 26.Xu L, Wang Y, Wang Y, et al. . A double-blinded randomized trial on growth and feeding tolerance with Saccharomyces boulardii CNCM I-745 in formula-fed preterm infants. J Pediatr 2016;92:296–301. doi:10.1016/j.jped.2015.08.013 [DOI] [PubMed] [Google Scholar]
- 27.Totsu S, Yamasaki C, Terahara M, et al. . Bifidobacterium and enteral feeding in preterm infants: cluster-randomized trial. Pediatr Int 2014;56:714–9. doi:10.1111/ped.12330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Awad H, Mokhtar H, Imam SS, et al. . Comparison between killed and living probiotic usage versus placebo for the prevention of necrotizing enterocolitis and sepsis in neonates. Pak J Biol Sci 2010;13:253–62. doi:10.3923/pjbs.2010.253.262 [DOI] [PubMed] [Google Scholar]
- 29.Braga TD, da Silva GA, de Lira PI, et al. . Efficacy of Bifidobacterium breve and Lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: a double-blind, randomized, controlled trial. Am J Clin Nutr 2011;93:81–6. doi:10.3945/ajcn.2010.29799 [DOI] [PubMed] [Google Scholar]
- 30.Chowdhury T, Ali MM, Hossain MM, et al. . Efficacy of probiotics versus placebo in the prevention of necrotizing enterocolitis in preterm very low birth weight infants: a double-blind randomized controlled trial. J Coll Physicians Surg Pak 2016;26:770–4. doi:2433 [PubMed] [Google Scholar]
- 31.Costalos C, Skouteri V, Gounaris A, et al. . Enteral feeding of premature infants with Saccharomyces boulardii. Early Hum Dev 2003;74:89–96. doi:10.1016/S0378-3782(03)00090-2 [DOI] [PubMed] [Google Scholar]
- 32.Dilli D, Aydin B, Fettah ND, et al. . The propre-save study: effects of probiotics and prebiotics alone or combined on necrotizing enterocolitis in very low birth weight infants. J Pediatr 2015;166:545–51. doi:10.1016/j.jpeds.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 33.Dutta S, Ray P, Narang A. Comparison of stool colonization in premature infants by three dose regimes of a probiotic combination: a randomized controlled trial. Am J Perinatol 2015;32:733–40. doi:10.1055/s-0034-1395473 [DOI] [PubMed] [Google Scholar]
- 34.Kanic Z, Micetic Turk D, Burja S, et al. . Influence of a combination of probiotics on bacterial infections in very low birthweight newborns. Wien Klin Wochenschr 2015;127:210–5. doi:10.1007/s00508-015-0845-0 [DOI] [PubMed] [Google Scholar]
- 35.Hays S, Jacquot A, Gauthier H, et al. . Probiotics and growth in preterm infants: A randomized controlled trial, PREMAPRO study. Clin Nutr 2016;35:802–11. doi:10.1016/j.clnu.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 36.Hussain M, Jabeen S, Subhani RUH. Role of probiotics in prevention of nectrotizing enterocolitis in preterm low birth weight neonates. Pak J Med Health Sci 2016;10:455–9. [Google Scholar]
- 37.Kitajima H, Sumida Y, Tanaka R, et al. . Early administration of Bifidobacterium breve to preterm infants: randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 1997;76:F101–7. doi:10.1136/fn.76.2.F101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Shimizu T, Hosaka A, et al. . Effects of bifidobacterium breve supplementation on intestinal flora of low birth weight infants. Pediatr Int 2004;46:509–15. doi:10.1111/j.1442-200x.2004.01953.x [DOI] [PubMed] [Google Scholar]
- 39.Manzoni P, Rinaldi M, Cattani S, et al. . Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA 2009;302:1421–8. doi:10.1001/jama.2009.1403 [DOI] [PubMed] [Google Scholar]
- 40.Mihatsch WA, Vossbeck S, Eikmanns B, et al. . Effect of Bifidobacterium lactis on the incidence of nosocomial infections in very-low-birth-weight infants: a randomized controlled trial. Neonatology 2010;98:156–63. doi:10.1159/000280291 [DOI] [PubMed] [Google Scholar]
- 41.Millar MR, Bacon C, Smith SL, et al. . Enteral feeding of premature infants with Lactobacillus GG. Arch Dis Child 1993;69:483–7. doi:10.1136/adc.69.5_Spec_No.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohan R, Koebnick C, Schildt J, et al. . Effects of Bifidobacterium lactis Bb12 supplementation on intestinal microbiota of preterm infants: a double-blind, placebo-controlled, randomized study. J Clin Microbiol 2006;44:4025–31. doi:10.1128/JCM.00767-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nandhini LP, Biswal N, Adhisivam B, et al. . Synbiotics for decreasing incidence of necrotizing enterocolitis among preterm neonates - a randomized controlled trial. J Matern Fetal Neonatal Med 2016;29:821–5. doi:10.3109/14767058.2015.1019854 [DOI] [PubMed] [Google Scholar]
- 44.Patole S, Keil AD, Chang A, et al. . Effect of Bifidobacterium breve M-16V supplementation on fecal bifidobacteria in preterm neonates--a randomised double blind placebo controlled trial. PLoS One 2014;9:e89511 doi:10.1371/journal.pone.0089511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reuman PD, Duckworth DH, Smith KL, et al. . Lack of effect of Lactobacillus on gastrointestinal bacterial colonization in premature infants. Pediatr Infect Dis 1986;5:663–8. doi:10.1097/00006454-198611000-00013 [DOI] [PubMed] [Google Scholar]
- 46.Rojas MA, Lozano JM, Rojas MX, et al. . Prophylactic probiotics to prevent death and nosocomial infection in preterm infants. Pediatrics 2012;130:e1113–20. doi:10.1542/peds.2011-3584 [DOI] [PubMed] [Google Scholar]
- 47.Romeo MG, Romeo DM, Trovato L, et al. . Role of probiotics in the prevention of the enteric colonization by Candida in preterm newborns: incidence of late-onset sepsis and neurological outcome. J Perinatol 2011;31:63–9. doi:10.1038/jp.2010.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roy A, Chaudhuri J, Sarkar D, et al. . Role of enteric supplementation of probiotics on late-onset sepsis by candida species in preterm low birth Weight neonates: a randomized, double blind, placebo-controlled trial. N Am J Med Sci 2014;6:50–7. doi:10.4103/1947-2714.125870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shadkam MN, Jalalizadeh F, Nasiriani K. Effects of probiotic lactobacillus reuteri (DSM 17938) on the incidence of necrotizing enterocolitis in very low birth weight premature infants. Iranian Journal of Neonatology 2015;6:15–20. [Google Scholar]
- 50.Stratiki Z, Costalos C, Sevastiadou S, et al. . The effect of a bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum Dev 2007;83:575–9. doi:10.1016/j.earlhumdev.2006.12.002 [DOI] [PubMed] [Google Scholar]
- 51.Samanta M, Sarkar M, Ghosh P, et al. . Prophylactic probiotics for prevention of necrotizing enterocolitis in very low birth weight newborns. J Trop Pediatr 2009;55:128–31. doi:10.1093/tropej/fmn091 [DOI] [PubMed] [Google Scholar]
- 52.Deshpande G, Rao S, Patole S. Probiotics for prevention of necrotising enterocolitis in preterm neonates with very low birthweight: a systematic review of randomised controlled trials. Lancet 2007;369:1614–20. doi:10.1016/S0140-6736(07)60748-X [DOI] [PubMed] [Google Scholar]
- 53.Battersby C, Longford N, Mandalia S, et al. . Incidence and enteral feed antecedents of severe neonatal necrotising enterocolitis across neonatal networks in England, 2012-13: a whole-population surveillance study. Lancet Gastroenterol Hepatol 2017;2:43–51. doi:10.1016/S2468-1253(16)30117-0 [DOI] [PubMed] [Google Scholar]
- 54.Rehan VK, Seshia MM, Johnston B, et al. . Observer variability in interpretation of abdominal radiographs of infants with suspected necrotizing enterocolitis. Clin Pediatr 1999;38:637–43. doi:10.1177/000992289903801102 [DOI] [PubMed] [Google Scholar]
- 55.Markiet K, Szymanska-Dubowik A, Janczewska I, et al. . Agreement and reproducibility of radiological signs in NEC using The Duke Abdominal Assessment Scale (DAAS). Pediatr Surg Int 2017;33:335–40. doi:10.1007/s00383-016-4022-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Di Napoli A, Di Lallo D, Perucci CA, et al. . Inter-observer reliability of radiological signs of necrotising enterocolitis in a population of high-risk newborns. Paediatr Perinat Epidemiol 2004;18:80–7. doi:10.1111/j.1365-3016.2003.00517.x [DOI] [PubMed] [Google Scholar]
- 57.Vongbhavit K, Underwood MA. Prevention of Necrotizing Enterocolitis Through Manipulation of the Intestinal Microbiota of the Premature Infant. Clin Ther 2016;38:716–32. doi:10.1016/j.clinthera.2016.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Warner BB, Deych E, Zhou Y, et al. . Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case-control study. Lancet 2016;387:1928–36. doi:10.1016/S0140-6736(16)00081-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjpo-2017-000066supp001.docx (16.4KB, docx)