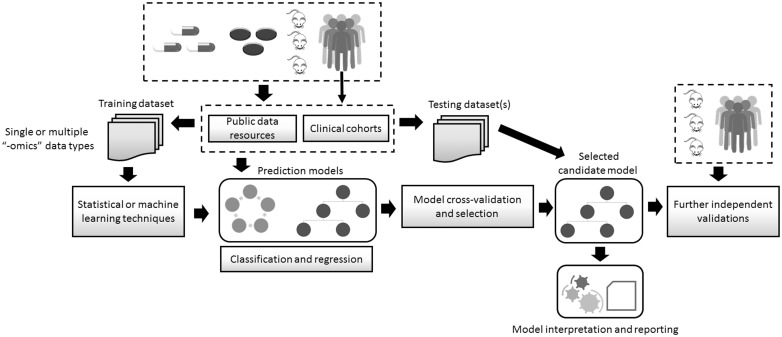
Figure 1.
Key steps in the development of computational models for predicting drug response. Data obtained from cell lines, animals or humans are stored in different data repositories, including public databases. These resources also include drug response information. Data sets are obtained to be subsequently used as training data sets, and may contain one or more types of ‘omics’ data, e.g. transcriptomics and DNA sequence. Such data are used as inputs to statistical or machine learning techniques. The prediction problem may be defined as either a classification or a regression problem, and a variety of techniques may be applied. The predictive performance of the models is assessed with cross-validation sampling techniques. The most-promising models are selected and evaluated using testing data sets, which were not used during the training phase. The model and its predictions undergo human expert interpretation and their reporting to stakeholders follows. Further independent validations using clinically relevant data are required to continue bridging the gap between the laboratory and the clinic.