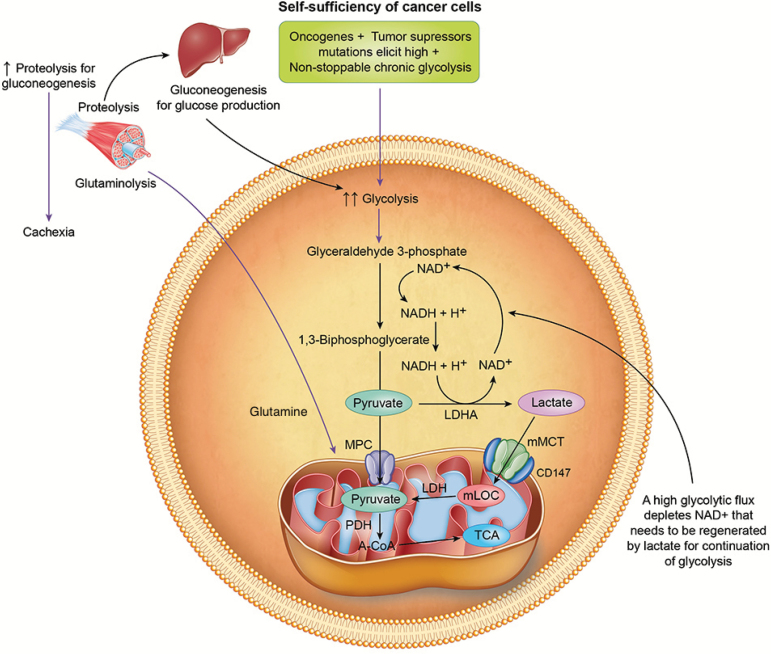
Figure 1.
Representation of self-sufficiency in cancer cells. Accelerated glycolysis elicited by oncogenes and tumor suppression mutations depletes nicotinamide adenine dinucleotide (NAD+). The reduction of pyruvate to lactate replenishes cytosolic levels of NAD+ and regulates the status of the equilibrium of the cytoplasmic redox pair (NADH/NAD+) for continuation of glycolysis. Lactate enters mitochondria and is oxidized to pyruvate and then acetyl CoA (A-CoA) through mitochondrial lactate oxidation complex (mLOC) comprised of mitochondrial monocarboxylate transporters (mMCT), its stabilizer, CD147, mitochondrial lactate dehydrogenase (LDH) and cytochrome oxidase (COx). Pyruvate can also enter mitochondria through mitochondrial pyruvate carrier (MPC) for oxidation to A-CoA. In glycolytic cancers, increased glycolysis is chronic which may deplete glycogen stores leading to increased proteolysis for gluconeogenesis, as well as for glutaminolysis to increase cytosolic pyruvate for lactate production. Chronic increased proteolysis for gluconeogenesis and glutaminolysis could explain cachexia in cancer.