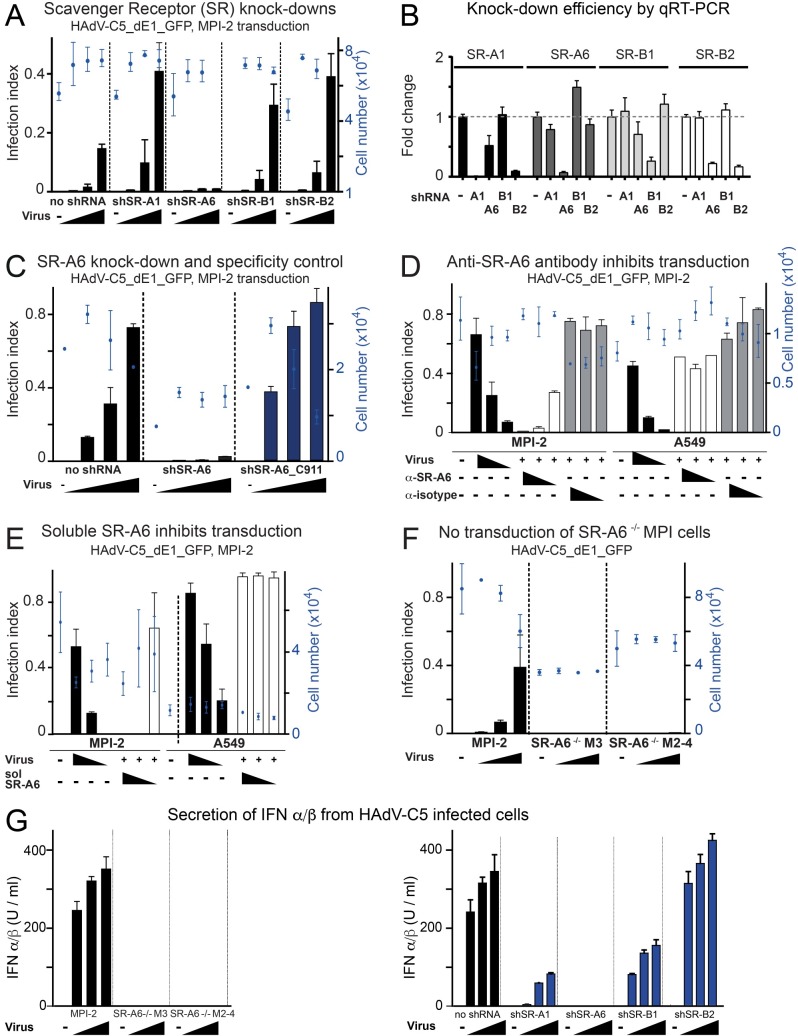
Fig 1. SR-A6 is required for HAdV-C5 infection of murine alveolar macrophage-like MPI-2 cells.
A) Transduction assay with HAdV-C5_dE1_GFP in MPI-2 cells expressing shRNAs against the scavenger receptors SR-A1, SR-A6, SR-B1 and SR-B2. Three different input amounts of HAdV-C5_dE1_GFP (~9765–39000 virus particles per cell) were used for the transduction. Transduction efficiency was scored at 20 h pi by measuring GFP signal intensity over nucleus, and is expressed as infection index (number of GFP positive nuclei/total number of nuclei). The values represent mean values from three technical replicates ± standard deviations. B) qRT-PCR control of knockdown efficiencies in shRNA-expressing cells. Transcripts analyzed are indicated on the upper part of the panel, and the annotation on x-axis indicates shRNA-expressing cell lysates used for analyses. The transcript levels are normalized to the wild type MPI-2 cells by three or two house-keeping genes. C) Transduction assay with HAdV-C5_dE1_GFP on MPI-2 cells expressing shRNA against SR-A6 or a control, non-targeting SR-A6_C911 shRNA. D) Transduction assay with HAdV-C5_dE1_GFP on MPI-2 and A549 cells after pre-incubation of cells with three different concentrations of anti-SR-A6 antibody ED31 or an isotype-matched control antibody. E) Transduction assay with HAdV-C5_dE1_GFP on MPI-2 and A549 cells after pre-incubation of virus with three different amounts of soluble mouse SR-A6. Input virus ~16700 virus particles per cell with the soluble SR-A6. F) Transduction assay with HAdV-C5_dE1_GFP on wild type MPI-2 and two independent SR-A6-/- MPI-cell lines. G) Secretion of IFN α/β from wild type, SR-A6 knock-out or scavenger receptor shRNA-expressing MPI-2 cells upon HAdV-C5 infection. Culture medium was collected from virus-infected cells 24h pi and titrated on a reporter cell line expressing the Firefly luciferase gene under the control of an IFN-inducible Mx2 promoter.