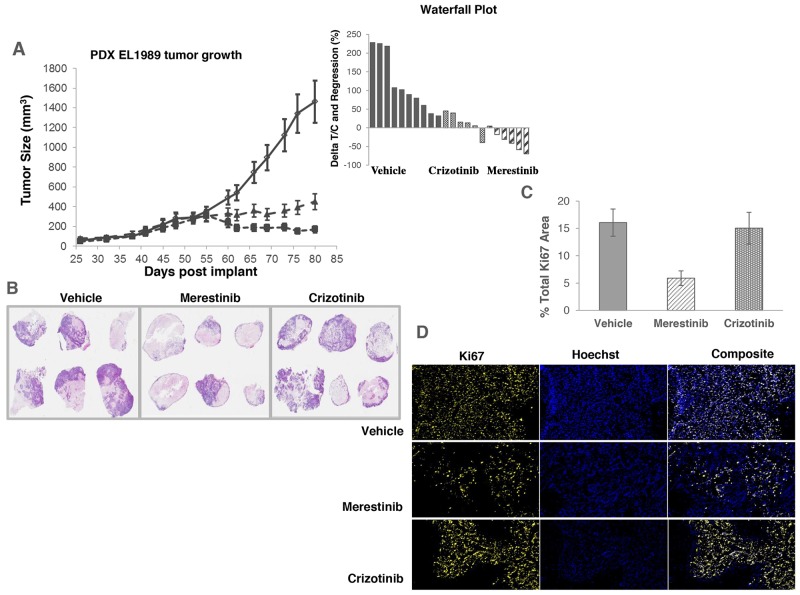
Figure 3. Anti-tumor activity of merestinib in patient-derived xenograft tumors (EL1989) bearing TPM3-NTRK1 fusion.
(A) Merestinib was dosed daily at 24 mg/kg (- -■- -), and crizotinib was dosed twice daily at 25 mg/kg (- -▲- -). All treatment cohorts began dosing on Day 52. Waterfall plot depicts individual animal tumor response to treatment as measured after 28 days of dosing (on Day 80). Graph bars below the x-axis indicate tumor regression. (B) Low magnification image (6x) of hematoxylin and eosin stained EL1989 PDX tumor histological sections grouped by treatment and harvested at the end of the study: Vehicle control tumors, merestinib treated tumors, crizotinib treated tumors. Viable tumor tissue stains light to dark purple, areas of necrosis stain as pale pink and areas of mucin accumulation are very pale or lack staining. Tumor viability, tumor necrosis and mucin accumulation scoring were performed by a board certified pathologist (KMC). Estimated % viable tumor tissue per cohort are as follows: Vehicle, mean 50%, range 5-90%; merestinib, mean 25%, range 5-70%; crizotinib, mean 50%, range 5-90%. Refer to Supplementary Table 2 for individual assessments. (C) Percent proliferating tumor cells by treatment based on Ki67 immunostaining and image analysis. Merestinib treated tumors (n=6) were significantly reduced (p=0.016) relative to vehicle (n=10). Crizotinib treated tumors were not significantly different (p=0.94). Five of six merestinib treated tumors showed thin rims of viable tumor tissue located mostly around the outer perimeter in comparison to the crizotinib treated cohort that displayed tumors with more abundant viable cells distributed throughout most tumor sections. Histological sections from vehicle control tumors were performed only on portions of the whole tumors rather than whole intact samples. Of these sections, viable cells were distributed throughout the tumors similar to crizotinib treated tumors. Tissue sections were stained with Ki67, and imaged and analyzed using an iCys laser scanning cytometer. Error bars denote SEM. (D) Representative image of the Ki67 immunostaining of the tumors from vehicle control, crizotinib and merestinib treated groups.