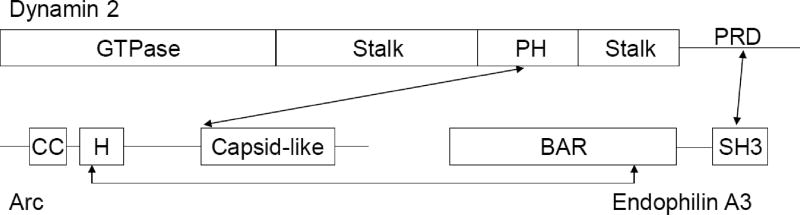
Figure 2. Scheme showing the interaction sites of Arc with Endophilin 3 and Dynamin 2.
Based on secondary structure predictions, Arc is divided into two domains connected by an extended intrinsically disordered region. The N-terminal half contains two stretches predicted to fold into α-helices, the first (~residues 30–60) with coiled-coil (CC) forming potential, the second (~residues 85–130; designated “H” in the figure) containing potential amphipathic segments. The C-terminal half of Arc contains a segment (residues 217–362) that is structurally similar to the retroviral Gag capsid [165]. The domains of dynamin and endophilin are described in the text.