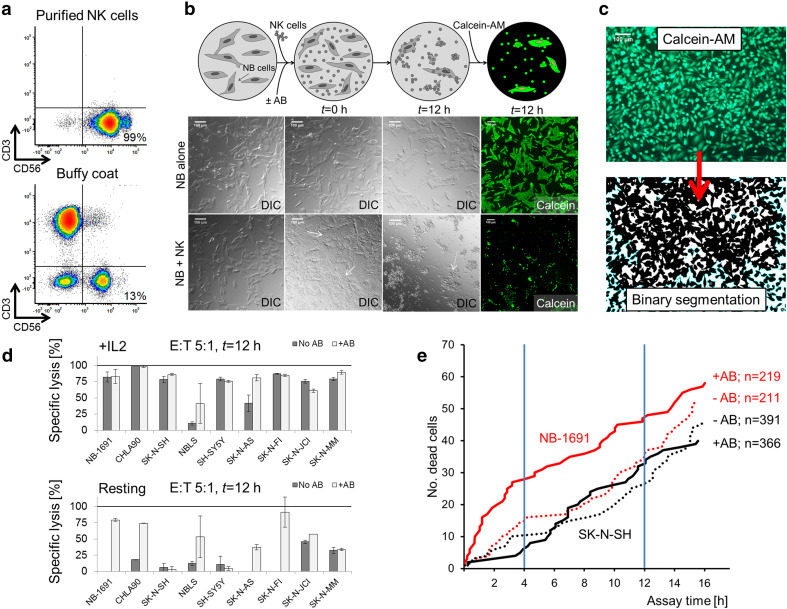
Fig. 1.
Development and validation of an assay to quantify NK cell-mediated cytotoxicity and ADCC in neuroblastoma cells. a Isolation of NK cells from peripheral whole blood yielded a highly purified CD56+/CD3− population (98%) compared with that of the buffy coat (13%). b Images produced in differential interference contrast (DIC) microscopy are shown. For the ADCC assay, neuroblastoma cells were plated into a 96-well plate and incubated with NK cells (solid arrow) ± Hu14.18K322A (t = 0 h). The number of neuroblastoma cells per well was counted to calculate the E:T ratio. After 12 h (t = 12 h), most NK cells were washed off with a small amount aggregated on neuroblastoma cells (dashed arrow). Viable cells were stained with calcein-AM and imaged by semiautomated microscopy. c Binary segmentation of the original image was performed with Fiji to determine cell confluence. d The effect of NK cell-mediated ablation (dark gray bars) and ADCC with Hu14.18K322A (light gray bars) was compared in resting and IL-2-activated NK cells (50 IU/mL) across nine neuroblastoma cell lines. The E:T ratio was 5:1 with a 12 h incubation time. e Two neuroblastoma cell lines were studied in co-culture by live-cell imaging. The E:T ratio was 5:1. The number of dead cells with ADCC and NK cell-mediated cytotoxicity was determined over time for NB-1691 (red line) and SK-N-SH (black line). Most significant differences between cell lines were seen with a 12-h incubation time.DIC differential interference contrast