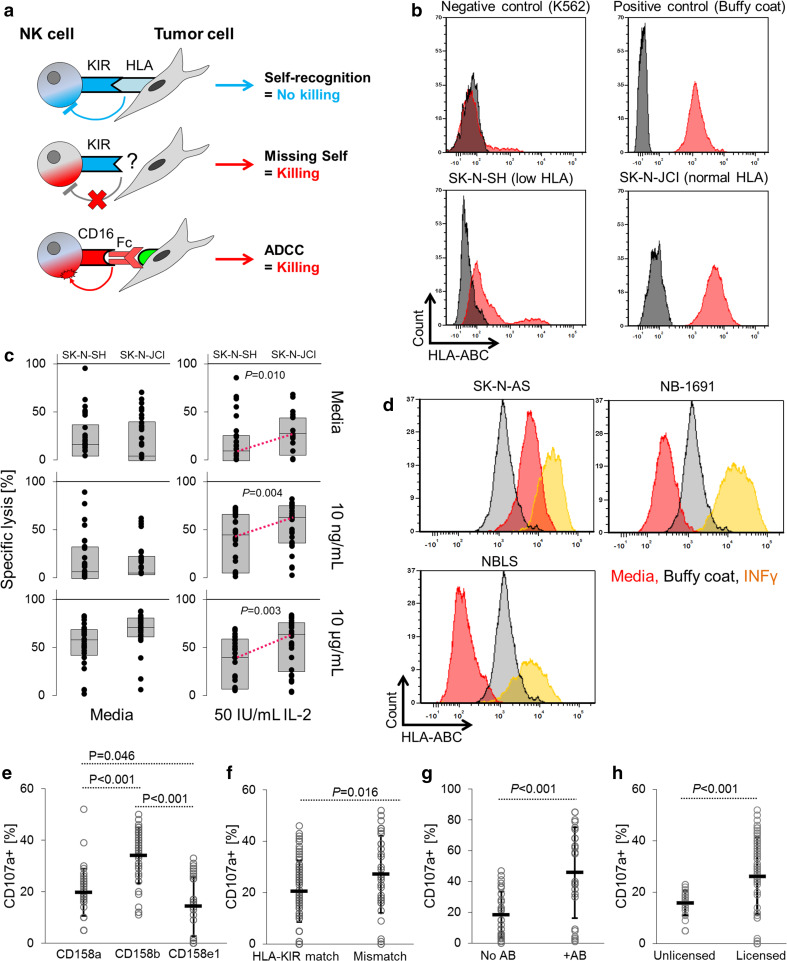
Fig. 4.
Comparison of ADCC and missing-self recognition for neuroblastoma cell ablation. a Schematic depicting HLA–KIR interactions and the principles of NK cell-mediated cytotoxicity. b The SK-N-SH neuroblastoma cell line demonstrated downregulation of HLA (red) surface expression, whereas the SK-N-JCI cell line expressed characteristic HLA levels. Isotype is shown in gray. K562 served as negative control and white blood cells from buffy coat as positive control. c Head-to-head comparison of susceptibility to NK cell-mediated cytotoxicity and ADCC. Activated NK cells induced more tumor cell death in SK-N-JCI than in SK-N-SH cells. Resting NK cells did not affect tumor cell death levels. d HLA expression was induced by interferon-γ treatment for 72 h and comparable to buffy coat. e–h Degranulation of NK cells from three donors was assessed by quantifying CD107a expression in single KIR-positive NK cell sub-populations after co-incubation with neuroblastoma cells that genotypically lacked the HLA class I molecules corresponding to CD158a (SK-N-AS), CD158b (NBLS), or CD158e1 (NB-1691). When stratified by KIR sub-population (e), presence of HLA–KIR mismatch between NK cells and tumor cells (f), use of antibody Hu14.18K322A (g), or licensing status and HLA–KIR mismatch (h), the greatest level of NK cell degranulation was noted in CD158b-expressing NK cells (e), in NK cells harboring KIRs that were mismatched against HLA expressed by the respective neuroblastoma cells (f), in the presence of antibody (g), and in licensed NK cells (h). KIR expression and HLA–KIR mismatch status remain statistically significant in a multiple regression analysis