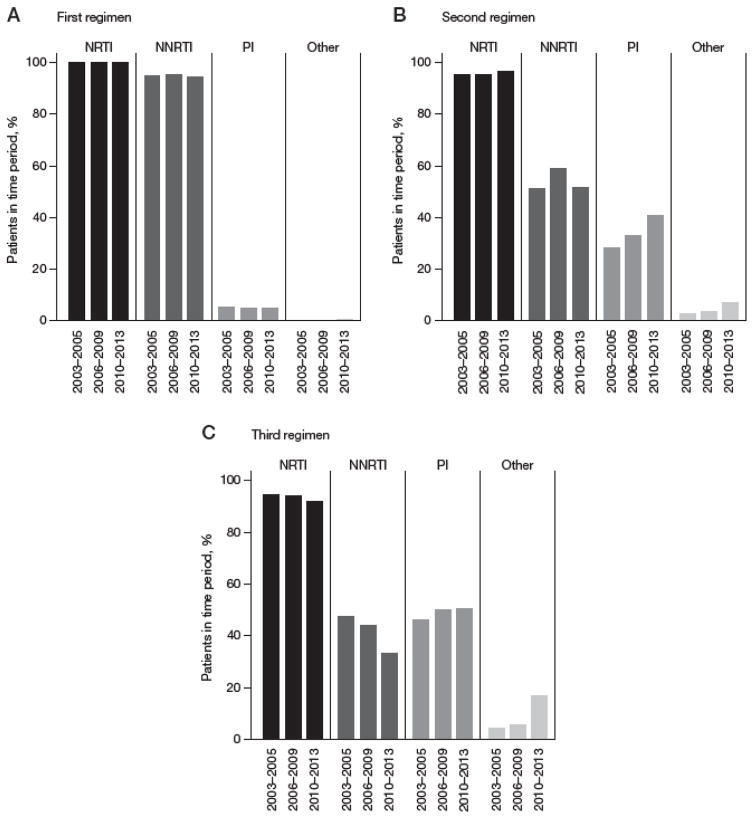
Figure 1. Proportion of patients initiating in each drug class, by year of ART initiation.
(A) First-line regimen (drugs initiated in other drug class for first-line regimen included: raltegravir [0.4% overall]; clinical trial drug [<0.1%]). (B) Second-line regimen (drugs initiated in other drug class for second regimen included: raltegravir [3.9%]; clinical trial drug [1.8%]). (C) Third-line regimen (drugs initiated in other drug class for third regimen included: raltegravir [8.5%]; cobicistat [0.5%]; elvitegravir [0.5%]; clinical trial drug [3.8%]). NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.