Figure 2. Bath application of CNO in the mPFC reduces glutamate release from MD pre-synaptic terminals.
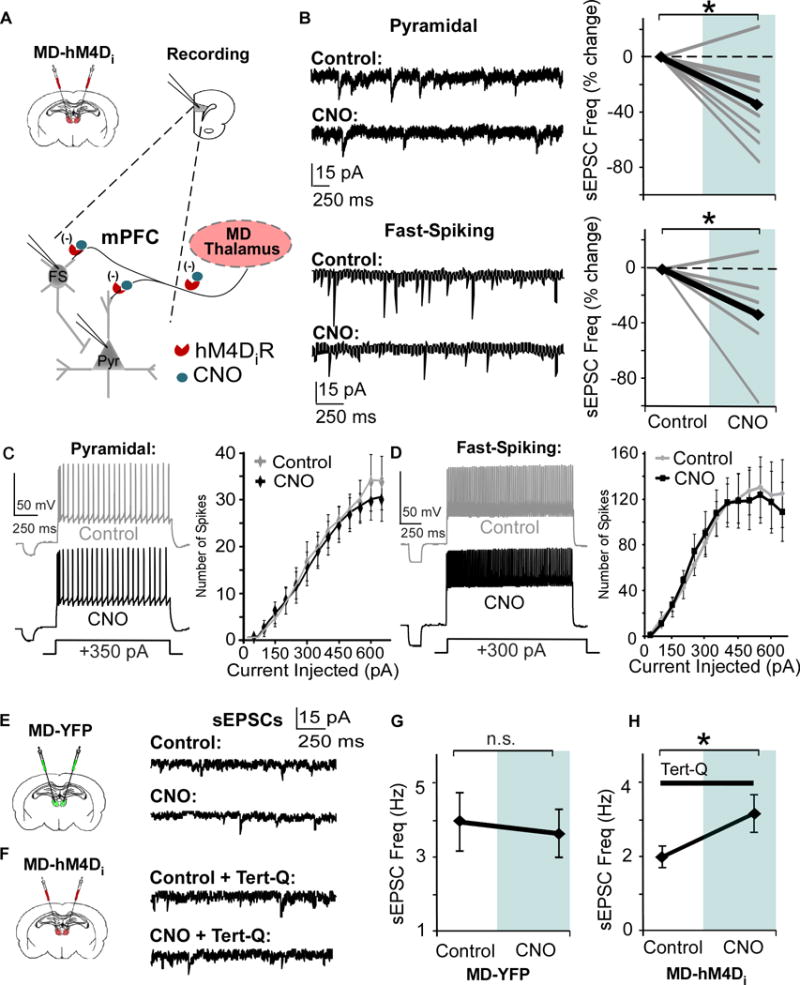
(A) Strategy for decreasing thalamic activity by inhibiting MD terminals in the mPFC. Graphs show a bilateral injection of the CaMKIIα-hM4D-mCherry or CaMKIIα-eYFP virus and patch-clamp recording in mPFC slices.
(B) Bath application of 1 μM CNO signficantly decreases sEPSC frequency in PNs and Fast-Spiking (FS) interneurons (Pyramidal, CNO = -34.8% ± 9.9; FS, CNO = -33.3% ± 12.7; paired t test, both * p < 0.05; pyramidal n = 9, FS n = 7, data shown as percent change from baseline).
(C) and (D) Spike number in response to depolarizing current injections is not altered by CNO in both PNs and FS interneurons (Repeated Measures ANOVA for final three current injections, Pyramidal, Control = 8.3 ± 0.76 pA; CNO = 8.3 ± 0.91 pA; paired t test, p > 0.05; FS, Control = 18.3 ± 1.64 pA; CNO = 16.6 ± 1.64 pA; paired t test, p > 0.05; pyramidal cells n = 9, FS interneurons n = 9).
(E) Left, animals received an injection of the control virus in the MD. Right, representative sEPSCs in mPFC pyramidal cells before and after bath application of CNO.
(F) Left, animals received an injection of the hM4D virus in the MD. Right, representative sEPSCs in mPFC pyramidal cells before and after CNO, with Tert-Q (100 nM) included in the bath.
(G) There was no difference in sEPSCs frequency before and after CNO in mPFC PNs in animals injected with the control virus in the MD (Control = 3.96 ± 0.79 Hz; CNO = 3.64 ± 0.66 Hz; paired t-test, p > 0.05).
(H) Including tert-Q in the bath solution occludes the decrease in sEPSC frequency (B), and sEPSC frequency increases with CNO in mPFC PNs in animals injected with the hM4D virus in the MD (Control = 2.00 ± 0.29 Hz; CNO = 3.17 ± 0.51 Hz; paired t-test, p > 0.05).
