Abstract
Objective
We aimed to study the effect of visual observation of bacterial growth from handprints on healthcare workers' (HCWs) compliance with hand hygiene (HH).
Settings
Medical and postoperative cardiac surgery units.
Design
Prospective cohort study.
Subject
The study included 40 HCWs.
Intervention
Each HCW was interviewed on 3 separate occasions. The 1st interview was held to obtain a handprint culture before and after a session demonstrating the 7 steps of HH using alcohol-based hand rub, allowing comparison of results before and after HH. A 2nd interview was held 6 weeks later to obtain handprint culture after HH. A 3rd interview was held to obtain a handprint culture before HH. One month before implementation of handprint cultures and during the 12-week study period, monitoring of HCWs for compliance with HH was observed by 2 independent observers.
Main Results
There was a significant improvement in HH compliance following handprint culture interview (p < 0.001). The frequency of positive cultures, obtained from patients with suspected healthcare-associated infections, significantly declined (blood cultures: p = 0.001; wound cultures: p = 0,003; sputum cultures: p = 0.005).
Conclusion
The visual message of handprint bacterial growth before and after HH seems an effective method to improve HH compliance.
1. Introduction
Annually about hundreds of millions of patients suffer from healthcare-associated infections HCAI worldwide [1]. One of the priorities of the patient safety goals is “First, do no harm” and to reduce the adverse health and social consequences of unsafe healthcare. The World Health Organization (WHO) contributes to this effort through the Patient Safety Programme with its First Global Patient Safety Challenge “Clean Care is Safer Care” (CCiSC), launched in 2005 and dedicated to the prevention of HCAI. One of the important recommendations for reducing HCAI is compliance with hand hygiene practices [2]. The CDC has published a guideline, interactive training and educational materials, and posters for HH compliance [3]. The interactive tools include a set of PowerPoint slides and speaker notes that provide background information on the importance of HH, indications on when to use HH practices and how to properly clean ones' hands, and educational/motivational programs [4]. Successful HH educational program has several key features: knowledge of healthcare workers' (HCWs) perceived importance of HH, monitoring and feedback of HH practices, practical education tools, role-modeling by senior staff, and supportive infrastructure and management [5].
Monitoring HCWs' compliance with HH practices is vital for evaluating whether interventions are successful. WHO recommends using a validated methodology for training observers to directly monitor HH using “My Five Moments for HH” [1]. Other methods for monitoring include patient observations, measuring of HH product consumption (either by volume of product used or through electronic counting devices), and electronic HH compliance monitoring systems [6]. Awareness of being watched can affect the usual behavior of individuals in many unpredictable ways other than simple work productivity effect. In the presence of auditing, HCWs might avoid activities that require HH. Measuring HH product use may overcome avoidance tactics. It is cheaper and generates continuous data to assess compliance of all clinicians without influencing patient care. Disadvantages include overestimation of product use through spillage, wastage, or use by visitors and nonclinical staff entering patient care areas. Electronic devices may overcome the Hawthorne and avoidance effects but are costly and are not widely used outside research studies [7].
We face the challenge of poor compliance with HH among HCWs despite posters, direct observation, and camera monitoring. We conducted this work to study the influence of visualization of bacterial growth from handprints, before and after use of alcohol-based disinfectant, on HCWs' compliance with HH.
2. Methodology
The study included 40 HCWs who were recruited from one medical and one postoperative cardiac surgical pediatric intensive care unit (PICU) at Cairo University Pediatric Hospital. We conducted an interventional program about the significance of alcohol-based HH in the two PICUs. The program was carried out in the following steps after this study was reviewed and approved by the local IRB.
HCWs were instructed to print both hands with no visible soiling on agar plates (15 × 15 cm) for 5 seconds (Hand Print 0-Before HH) [8] and the plate was then incubated immediately.
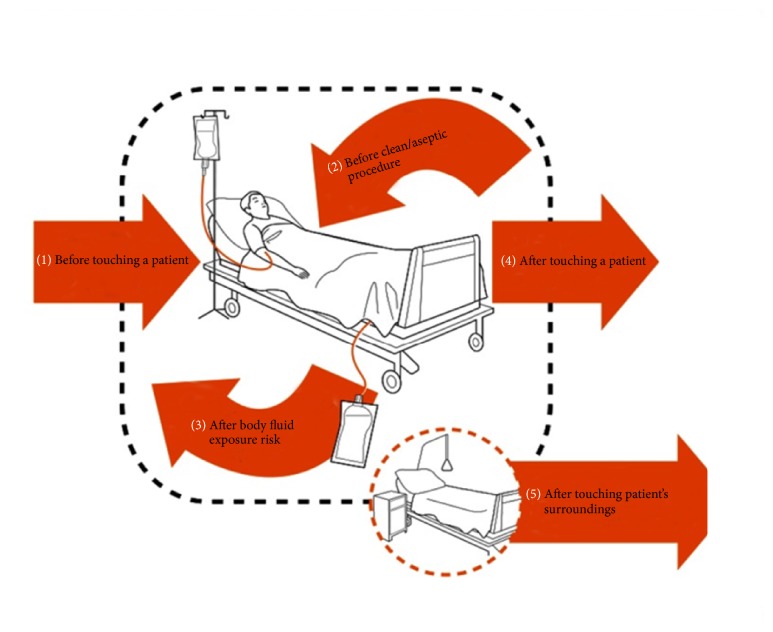
The 7 steps of proper HH technique were demonstrated by a mentor physician from the PICU and infection control team, using alcohol-based liquid hand disinfectant and providing advice on the occasions for HH “My Five Moments for HH” (Figure 1) [1].
Figure 1.
“My Five Moments for Hand Hygiene,” WHO, 2009.
Each HCW was monitored while performing proper HH.
A second handprint on agar plate was obtained after proper HH, just after drying of hands (Hand Print 0-After HH).
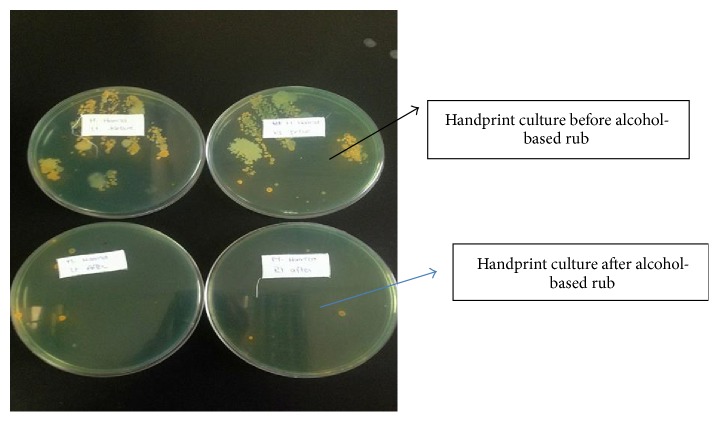
The HCWs were interviewed 48 hours later to provide them with the results of their handprint cultures, before and after HH, by making them inspect the agar plates and discussing the effect of HH on microbial growth on the plates (Figure 2).
Figure 2.
The handprints of one of HCWs before and after hand hygiene by alcohol-based disinfectant.
Data were collected from each HCW including age, gender, occupation, working hours per week, nurse to patient ratio, dominant hand, and wearing long sleeves or not.
Handprint cultures were obtained from HCWs 6 weeks later after HH was applied (Hand Print-6 weeks-After HH). The HCWs were interviewed 48 hours later about the results of their handprint culture.
After another 6 weeks, handprint cultures were obtained from each HCW without prior HH, with no visible soiling and not immediately following any procedure involving patient contact (Hand Print-12 weeks-Before HH). Again the HCWs were interviewed 48 hours later about the results of their handprint culture.
One month before implementation of handprint cultures and during the 12-week study period, monitoring of HCWs for compliance with HH was observed by 2 independent observers using the WHO checklist for HCWs HH compliance [1].
2.1. Sample Processing
After collection, samples were incubated aerobically for 24 hours. After incubation the plates were observed for colony morphology and colony count, with microscopic examination of gram stained films. Isolates, if any, were identified by standard microbiologic techniques, namely: Gram staining, colony characteristics, and biochemical properties, according to [9]. Cefoxitin disc (30 μg) was used to screen methicillin-resistant Staphylococcus aureus (MRSA). Transient flora was defined as any pathogen other than coagulase-negative staphylococci (CoNS), Corynebacterium sp., Micrococcus sp., or Bacillus sp. Gram-negative lactose nonfermenters included Acinetobacter, Bordetella, Burkholderia, Legionella, Moraxella, Pseudomonas, and Stenotrophomonas. Gram-negative lactose fermenters included Enterobacter spp., Escherichia coli, and Klebsiella.
2.2. Statistical Analysis
Data were analyzed by the Statistical Package for Social Sciences (SSPS version 21). Continuous variables were compared with the unpaired t-test. Categorical variables were compared with X2 and Z-score tests. Probability variables were reported, and those less than 0.05 were considered statistically significant.
3. Results
Among the studied cohort, 22 HCWs (55%) were females. The mean working hours per/week were 52 ± 20 hours. The characteristics of the study population are shown in Table 1.
Table 1.
Microbial growth over the dominant and nondominant hand of the healthcare workers before applying hand hygiene (Hand Print 0-Before HH).
| CFU per type of Bacteria | Dominant hand Hand Print 0-Before HH |
Nondominant hand Hand Print 0-Before HH |
p value |
|---|---|---|---|
| Total CFU per HCW hand | |||
| Median (min–max) | 36 (0–185) | 32 (0–117) | 0.3 |
| Transient bacteria | |||
| CFU per HCW hand | |||
| Median (min–max) | 22 (2–180) | 25 (1–115) | 0.5 |
| Resident bacteria | |||
| CFU per HCW hand | |||
| Median (min–max) | 13 (2–50) | 5 (1–40) | 0.3 |
CFU= colony forming unit; HH = hand hygiene, HCW = healthcare worker.
On the first occasion (Hand Print 0-Before HH), the hands of the 40 HCWs showed no statistically significant difference between dominant and nondominant hands as regards the type or frequency of microbial growth (Table 1). Among the transient pathogenic bacteria: Staphylococcus aureus was the most prevalent (38%), one-quarter of which was MRSA. Other detected pathogenic organisms were Gram-negative non-lactose fermenting (25%) and Gram-negative lactose fermenting bacteria (10%). Among Gram-negative lactose nonfermenting organisms, Pseudomonas constituted 0.8%. Resident flora included CoNS (19%) and anthracoids (6%) (Table 3). After alcohol hand rub (Hand Print 0-After HH), there was a significant decline in the total number of colonies detected in HCWs' handprint cultures (p = 0.0006). A decline was noted in all of the organisms detected in the cultures (Table 2).
Table 3.
Comparison of results of Hand Print 0-After HH and Hand Print-6 weeks-After HH.
| CFU according to microorganisms | Hand Print 0-After HH | Hand Print-6 weeks-After HH | p value |
|---|---|---|---|
| Median number of CFU/dominant hand/HCW (min–max) for all bacteria | 2 (1–51) | 5 (1–110) | 0.07 |
| Total number of CFU/dominant hands of the 40 HCWs | 134 | 346 | 0.608 |
| Transient bacteria/HCWs; median number of CFU/dominant hand/HCWs (%) | |||
| Staphylococcus aureus; N (%) | 88 (65.7) | 155 (35) | <0.0001 |
| Gram negative non-lactose fermenters; N (%) | 22 (16) | 75 (17) | 0.13622 |
| Gram negative lactose fermenters; N (%) | 7 (5) | 0 | <0.0001 |
| Pseudomonas; N (%) | 3 (2.24) | 0 | 0.0251 |
| Resident bacteria/HCWs; median number of CFU/dominant hand/HCWs (%) | |||
| Staph. CoNS; N (%) | 12 ( 9 ) | 197 (45) | <0.0001 |
| Anthracoids; N (%) | 2 (1.5) | 9 (2) | <0.0001 |
Table 2.
Comparison between Hand Print-0 cultures before and after applying hand hygiene.
| Number of CFU according to the type of bacteria | Hand Print 0-Before HH | Hand Print 0-After HH | p value |
|---|---|---|---|
| Median number of CFU/dominant hand/HCW (min–max) for all bacteria | 25 (1–185) | 2 (1–50) | 0.0006 |
| Total number of CFU/dominant hands of the 40 HCWs | 1444 | 134 | 0.003 |
| Transient bacteria/HCWs; median number of CFU/dominant hand/HCWs (%) | |||
| Staphylococcus aureus; N (%) | 560 (38.7) | 88 (65) | 0.0001 |
| Gram negative non-lactose fermenters; N (%) | 364 (25) | 22 (16) | 0.0001 |
| Gram negative lactose fermenters; N (%) | 147 (10) | 7 (5) | 0.0001 |
| Pseudomonas; N (%) | 12 (0.8) | 3 (2.24) | 0.0001 |
We compared the Hand Print 0-After HH to Hand Print-6 weeks-After HH. The median number of bacteria/HCW hand increased but did not reach statistical significance (p = 0.07) (Table 3).
There was a reduction in the percent of S. aureus on the hands of HCWs before HH when comparing Hand Print 0-Before HH to Hand Print 12 weeks-Before HH (Table 4). On the other hand, resident commensal bacteria, namely, CoNS increased significantly (p < 0.0001).
Table 4.
Comparison between Hand Print 0-Before HH and Hand Print-12 weeks-Before HH.
| CFU per type of Bacteria | Hand Print 0-Before HH | Hand Print-12 weeks-Before HH | p value |
|---|---|---|---|
| Median number of CFU/dominant hand/HCW (min–max) for all bacteria | 25 (1–185) | 20 (0–200) | 0.66 |
| Total number of CFU/dominant hands of the 40 HCWs | 1444 | 1915 | 0.822 |
| Transient bacteria/HCWs; median number of CFU/dominant hand/HCWs (%) | |||
| Staphylococcus aureus; N (%) | 560 (38.7) | 275 (18) | <0.000 |
| Gram negative non-lactose fermenters; N (%) | 364 (25) | 173 (9) | <0.001 |
| Gram negative lactose fermenters; N (%) | 147 (10) | 19 (0.99) | <0.0001 |
| Resident bacteria/HCWs; median number of CFU/dominant hand/HCWs (%) | |||
| Staph. CoNS; N (%) | 277 (19) | 963 (50) | <0.0001 |
| Anthracoids; N (%) | 89 (6) | 64 (4) | 0.603 |
3.1. Observation of Compliance of HCWs with HH
Before the start of this intervention, we observed 147 opportunities for HH among HCWs, 78% of the opportunities were missed. After implementation of our handprint culture training we observed another 147 opportunity for HH. There was a significant reduction in the percentage of missed opportunities (30% versus 78%, p < 0.0001) (Figure 3). The most frequently missed opportunity for HH, after the intervention, was after touching the patient surroundings (Figure 4).
Figure 3.
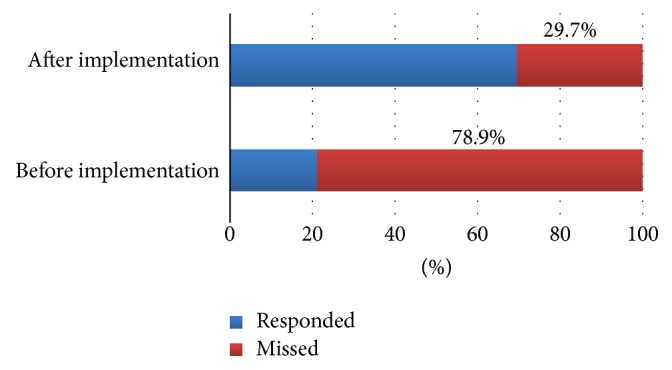
The frequency of missed opportunity before and after implementation of handprint cultures.
Figure 4.
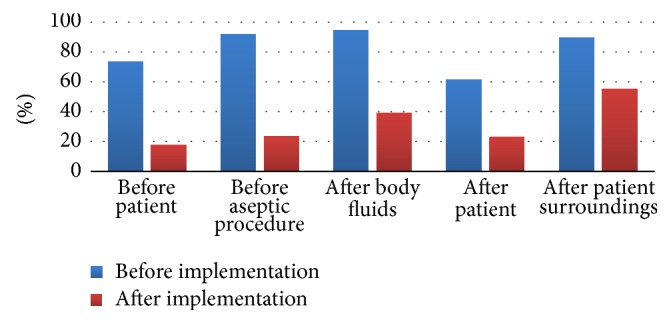
Compliance with hand hygiene before and after intervention according to the 5 moments of hand hygiene.
3.2. Change in the Frequency of Positive Bacterial Cultures in PICUs
The results of different patients' cultures, taken at least 72 hours after admission, that is, hospital associated infections, were reviewed from the hospital database 6 months before and 6 months after intervention. Positive cultures were reduced from 51% to 37% (p = 0.001) (Table 2).
4. Discussion
Our study has shown that the use of handprint cultures before and after HH, as a visual tool, to convince the HCWs about the effectiveness of alcohol-based hand rubs on the bacterial load in the hands, could effectively increase their compliance. As observed, the missed opportunities according to “My Five Moments of HH” declined from 78% to 30% (p value < 0.001) with a parallel reduction in rates of positive cultures. This technique was adapted from Yamamoto et al. [8], who used this method for psychiatric hospital staff, which was effective in promoting awareness of the importance of HH and encouraged appropriate use of hand antiseptics measured by its consumption. Simple messages using appeals to social situations and to ego (self-efficacy) were rated as most likely to increase HH compliance [10].
HH using alcohol-based disinfectant could reduce the colony forming units (CFU) on hands of the HCWs. The CDC task force for HH [4] reported that the in vivo antimicrobial activity of alcohols effectively reduces bacterial counts on the hands.
In the present study, no difference was detected between the dominant and the nondominant hand on bacterial growth. In a previous study, the recovery rate of Gram-negative bacteria was higher on the nondominant hand than on the dominant hand but the difference was not statistically significant (p = 0.21) [11].
Staphylococcus aureus was the most prevalent on the hands of HCWs (38%), one-quarter of which was MRSA (almost 10% of the total). This is higher than the prevalence reported on the basis of 145 papers published between 1980 and March 2010, which was around 5%. It is assumed that MRSA rates will be higher when HCWs comply poorly with HH and contact precautions, as they are not fully aware of the threat of the bacteria load [12, 13].
The results of Hand Print-6 weeks-After HH showed a lower frequency of growth of pathogenic microorganisms compared to Hand Print 0-After HH except for the Gram-negative non-lactose fermenters. Also a third Hand Print-12 weeks-Before HH demonstrated reduction in the number of transient bacteria in the hands of HCWs compared to Hand Print 0-Before HH. The increased compliance could reduce the pathogenic microbial load on the hands of HCWs. But the CoNS increased probably because of inadequate technique.
Periodic technique simulation for the HCWs is important for the adequacy of their HH and not only their personal motivation. After a single simulation education session, critical care nurses' knowledge of and adherence to current HH guidelines remained below targeted behavior rates [14]. The effectiveness of HH as an infection control measure relates not only to the frequency with which it is carried out, but also to how effectively it is undertaken [15]. Figure 3 shows the frequency of increased compliance before and after the application of the intervention.
The most frequently missed indication was handwashing after touching patient surroundings (Figure 4). This is consistent with the results obtained from studies in different ICUs where compliance with moment 5 (after touching patient surroundings) was the lowest [16, 17]. Although it is ideal to observe all five hand hygiene moments recommended by the WHO, it is often not feasible to observe practices performed at the bedside. Therefore, some hospitals choose to observe HH before and after patient contact. It is reported that this before/after-contact monitoring method can be used as a surrogate indicator of rates based on all five moments, if certain conditions are met [18].
The rates of positive cultures in the PICU, before and after handprint culture implementation, were compared as an indirect expression of the efficacy of the intervention. It was clear that influencing the behavior of HCWs about HH by the handprint culture interview was significantly contributing factor not only in decreasing the pathogenic microbiological load on their hands but also in decreasing the rates of hospital associated infections.
5. Conclusion
Visual messages about the burden of bacterial growth on the hands of HCWs are important for enforcing HH and complying with “My Five Moments for HH”.
6. Study Limitations
Remote followup of handprint culture after several weeks to evaluate the possible Hawthorne effect during the intervention period is a limitation in the study. Another set of cultures after another 3–6 months to evaluate the burden of pathogenic bacteria in the hands of healthcare staff and to remind the healthcare staff that the value of HH on self and patient safety is needed for better patient safety.
Acknowledgments
The authors would like to express great thanks to Jun Moryama and the whole members of the NCGM in Tokyo who taught them the educational value of handprint cultures.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.World Health Organization. WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge. 2009. [PubMed]
- 2.Consensus Measurement in Hand Hygiene Project Expert Advisory Panel. Measuring hand hygiene adherence: overcoming the challenges. Oakbrook Terrace, Ill, USA: The Joint Commission; 2009. [Google Scholar]
- 3.Centers for Disease Control and Prevention. Hand Hygiene in Health Care Settings: Training. May 19, 2011. https://www.cdc.gov/handhygiene%E2%80%8B/training/interactiveEducation/ [Google Scholar]
- 4.Centers for Disease Control and Prevention. Guideline for Hand Hygiene in Health-Care Settings: Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA HandHygiene Task Force. Vol. 51. MMWR; 2002. [Google Scholar]
- 5.Gould D. J., Moralejo D., Drey N., Chudleigh J. H. Interventions to improve hand hygiene compliance in patient care. Cochrane Database of Systematic Reviews. 2010;9 doi: 10.1002/14651858.CD005186.pub4.CD005186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mertz D., Dafoe N., Walter S. D., Brazil K., Loeb M. Effect of a multifaceted intervention on adherence to hand hygiene among healthcare workers: A cluster-randomized trial. Infection Control and Hospital Epidemiology. 2010;31(11):1170–1176. doi: 10.1086/656592. [DOI] [PubMed] [Google Scholar]
- 7.Gould D. J., Creedon S., Jeanes A., et al. Impact of observing hand hygiene in practice and research: a methodological reconsideration. The Journal of Hospital Infection. 2017;96(199) doi: 10.1016/j.jhin.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto Y., Iwawaki Y., Murota M., Takishita Y. Usefulness of training in hand hygiene using the full-hand touch plate method for hospital staff: Practice at a psychiatric hospital. Japanese Journal of Infection Prevention and Control. 2015;30(4):281–287. doi: 10.4058/jsei.30.281. [DOI] [Google Scholar]
- 9.Carroll K. C., Patel R. Manual of clinical microbiology. In: Jorgensen J., Pfaller M. A., Carroll K. C., Landry M. L., editors. American Society for Microbiology. 11th. Chapter 4. 2015. pp. 29–43. [Google Scholar]
- 10.Taylor R. E. Perceived effectiveness of messages promoting hand hygiene. American Journal of Infection Control. 2017;45(3):314–316. doi: 10.1016/j.ajic.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Gudza-Mugabe M., Magwenzi M. T., Mujuru H. A., Bwakura-Dangarembizi M., Robertson V., Aiken A. M. Effect of handrubbing using locally-manufactured alcohol-based handrubs in paediatric wards in Harare, Zimbabwe. Antimicrobial Resistance and Infection Control. 2017;6(1, article no. 8) doi: 10.1186/s13756-016-0166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albrich W. C., Harbarth S. Health-care workers: source, vector, or victim of MRSA? The Lancet Infectious Diseases. 2008;8(5):289–301. doi: 10.1016/s1473-3099(08)70097-5. [DOI] [PubMed] [Google Scholar]
- 13.Hawkins G., Stewart S., Blatchford O., Reilly J. Should healthcare workers be screened routinely for meticillin-resistant Staphylococcus aureus? A review of the evidence. Journal of Hospital Infection. 2011;77(4):285–289. doi: 10.1016/j.jhin.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 14.Jansson M. M., Syrjälä H. P., Ohtonen P. P., et al. Simulation education as a single intervention does not improve hand hygiene practices: a randomized controlled follow-up study. American Journal of Infection Control. 2016;44(6):625–630. doi: 10.1016/j.ajic.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 15.Eksi F., Mehli M., Akgun S., Bayram A., Balci I., Aydin N. Evaluation of two different hand hygiene procedures during routine patient care. Journal of International Medical Research. 2010;38(6):2084–2092. doi: 10.1177/147323001003800624. [DOI] [PubMed] [Google Scholar]
- 16.Harbarth S., Pittet D., Grady L., Goldmann D. A. Compliance with hand hygiene practice in pediatric intensive care. Pediatric Critical Care Medicine. 2001;2(4):311–314. doi: 10.1097/00130478-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Chavali S., Menon V., Shukla U. Hand hygiene compliance among healthcare workers in an accredited tertiary care hospital. Indian Journal of Critical Care Medicine. 2014;18(10):689–693. doi: 10.4103/0972-5229.142179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakamoto F. Hand hygiene aadherence monitoring: how to capture and improve the true apos; rates. Japanese Journal of Infection Prevention and Control. 2017;32:1–5. [Google Scholar]