Abstract
In female mice, the expression of receptive lordosis behavior requires estradiol and progesterone actions in the nervous system; however, the contribution of these hormones to females’ motivation to seek out male pheromones is less clear. In an initial experiment, sexually naïve ovary-intact female mice preferred to investigate (make nasal contact with) testes-intact male as opposed to estrous female urine, provided they were in vaginal estrus. In a second experiment, groups of sexually naïve and mating-experienced, ovariectomized females were tested for urinary pheromone preference first without and then with ovarian hormone replacement. Without hormone replacement, sexually naïve ovariectomized females showed no preference for male over female urinary pheromones whereas mating-experienced females preferred to investigate male pheromones. Ovariectomized females in both groups preferred male over female urine after sequential s.c. injections with estradiol benzoate followed 2 days later with progesterone and after prolonged (7 days) exposure to estradiol alone. Our results indicate that in sexually naïve female mice estradiol, perhaps aided by progesterone, is required to motivate a preference to seek out male pheromones whereas after mating experience females’ preference to investigate male pheromones persists even in the absence of ovarian hormone action.
Keywords: female sexual behavior, odor preference, pheromone, progesterone
Introduction
In female mice, the expression of lordosis, the reflexive, arched-back posture displayed in response to male mounts, depends on the sequential forebrain actions of the ovarian hormones estradiol (E2) and progesterone (P). Thus, females that have undergone bilateral ovariectomy, and therefore lack circulating ovarian hormones, fail to show lordosis in response to male mounts (Thompson and Edwards 1971). Ovariectomized female mice require injections of both estradiol benzoate (EB) and P in the correct sequence (treatment with EB followed 2 days later by P) to express any lordosis in response to the receipt of male mounts (Edwards 1970; Thompson and Edwards 1971) and in order to retain a seminal plug in the vagina after spending a night with a stud male (Ring 1944). This sequential regimen of ovarian hormone treatments is intended to approximate the natural variations in circulating levels of ovarian hormones that occur during the first half of the 4–5 day estrous cycle of ovary-intact female mice. Circulating levels of E2 surge on the night of vaginal proestrus just before the preovulatory surge in luteinizing hormone (LH) (Walmer et al. 1992; Wood et al. 2007). There is also a small peak in serum P concentrations on the night of proestrus (Walmer et al. 1992) that is essential for the expression of lordosis. Considerably higher serum concentrations of P are found 1–2 days later, at the time of vaginal diestrus (Fata et al. 2001; Wood et al. 2007; Joshi et al. 2010). Whereas both E2 and P actions are required for the expression of receptive lordosis behavior in female mice (Ring 1944; Edwards 1970; Thompson and Edwards 1971), the role of these 2 ovarian hormones in females’ motivation to seek out urinary pheromones produced by testes-intact male mice is less clear.
Male mouse urine contains nonvolatile compounds including the major urinary protein (MUP) Darcin that attracts female mice (Roberts et al. 2010, 2012). These nonvolatile urinary cues are detected by sensory neurons in the vomeronasal organ (VNO) of the accessory olfactory system (AOS) (Chamero et al. 2012). These sensory neurons project to the accessory olfactory bulb (AOB), which sends mitral/tufted cell projections to the medial amygdala (Mea) and to the bed nucleus of the stria terminalis (BNST) (Kevetter and Winans 1981; Restrepo et al. 2004). Forebrain regions critical for reproduction, such as the ventromedial hypothalamus (VMH) and the medial preoptic area (MPA), process pheromonal inputs via axonal projections from the Mea (Choi et al. 2005; Pardo-Bellver et al. 2012). The morphology and function of neurons in several segments of the female AOS are affected by ovarian sex steroids. An early study (Halem et al. 1999) showed that the ability of male pheromones to stimulate expression of the immediate-early gene, c-fos, in VNO sensory neurons of ovariectomized female mice was augmented by treatment with EB. By contrast, more recent experiments using in vitro preparations of VNO sensory neurons from mice of both sexes showed that E2 and P actually reduced indices of neuronal activity otherwise activated by putative male urinary pheromones (Cherian et al. 2014; Dey et al. 2015). Dendritic spine density was highest in the posterior Mea of sexually naïve, ovary-intact female rats in diestrus compared to the other stages of the estrous cycle (Rasia-Filho et al. 2004), while treatment with ovarian hormones stimulated dendritic spine density in the Mea of ovariectomized female rats (de Castilhos et al. 2008). Finally, administering EB to ovariectomized female mice augmented their ability to detect dilute male urine in a food-motivated sand digging task (Sorwell et al. 2008). Taken together, these diverse findings suggest that ovarian hormones may modulate the responsiveness of the AOS to male pheromones and raise the question whether ovarian hormone actions are required in order for female mice to display a preference to investigate nonvolatile urinary pheromones from male versus female conspecifics.
A recent study (Dey et al. 2015) found that sexually naïve, ovary-intact female mice preferred to investigate either the high molecular weight fraction of male urine or recombinant male MUPs instead of phosphate buffered saline, provided they were in vaginal estrus as opposed to diestrus. This study appears to disagree with the outcome of other olfactory preference studies in which female subjects were allowed to choose between pheromonal stimuli from the 2 sexes. Moncho-Bogani et al. (2002) found that ovary-intact female mice preferred to investigate (make nasal contact with) male as opposed to female soiled bedding even when they were in vaginal diestrus. In another recent study (Nomoto and Lima 2015), ovary-intact female mice preferred to make nasal contact with the body of a testes-intact male as opposed to a female regardless of whether subjects were in vaginal estrus or diestrus. A more rigorous way to assess the role of ovarian hormones in females’ pheromone preference is to ovariectomize female mice and then test their pheromone preferences before and after administration of EB and P. Moncho-Bogani et al. (2004) found that chemically naïve (not previously exposed to male odors) ovariectomized female mice preferred to investigate male as opposed to female soiled bedding regardless of whether they received no hormone replacement (i.p. injections of sunflower oil vehicle), EB alone, P alone, or a sequence of EB followed 2 days later by P. In light of the discrepancies among these various prior experiments, we decided to re-examine the question of whether ovary-intact female mice that lack prior mating experience prefer to make nasal contact with male versus female urine and whether a preference for male urine varies across the ovarian cycle as monitored using vaginal lavage.
Another factor that could contribute to females’ preference to seek out male pheromones is their prior mating experience with a male. During mating, females are exposed to a wide range of chemosensory, tactile, visual, and auditory stimuli that could alter their later motivation to seek out male pheromones. Numerous previous studies have shown that ovariectomized, sexually naïve female mice treated sequentially with injections of both EB and P show low levels of lordosis when first paired with a male, but show higher levels of lordosis after repeated pairings (Edwards 1970; Laroche et al. 2009a, 2009b; Bonthuis et al. 2011; Ismail et al. 2011), but the role of ovarian hormones in mediating the preference for male odor cues in female mice after sexual experience is unknown. Therefore, in a second experiment, the preference to make nasal contact with male versus female urinary odors was tested in both sexually naïve and in mating-experienced female mice that had been ovariectomized and given no hormone replacement followed later by treatment with E2 either without or with subsequent, sequential administration of P.
Materials and methods
Subjects
Female (n = 34) and male (n = 9) C57BL/6J mice were purchased for use in these experiments (age 5–7 weeks; Charles River Laboratories). Females were group housed (3–5 mice per cage). Males were initially group housed (4 males per cage) before receiving sexual experience whereupon they were singly housed. Mice were maintained on a reversed 12:12 h light:dark cycle with water and food available ad libitum. All procedures were approved by the Boston University Charles River Campus Institutional Animal Care and Use Committee.
Surgery
In Experiment 2, female mice (n = 18) were bilaterally ovariectomized under 2% isoflurane anesthesia and were given s.c. injections of analgesic (carprofen, 5 mg/kg) on the day of surgery and for 2 subsequent days. Mice were allowed to recover from surgery for 3 weeks before the start of behavioral testing. A separate group of female mice (n = 6) also received bilateral ovariectomies and were later brought into behavioral estrus with s.c. injections of EB followed by 2 days later by P to collect urine for odor preference testing and to give stud males sexual experience. Urine collection began 1 week after ovariectomy. Stud males were considered to be sexually experienced after 2 overnight pairings with an estrous female mouse.
Pheromonal stimuli
Urine was collected from sexually naïve, testes-intact male (IMU) (n = 6) and from estrous female (EFU) (n = 6) mice for use in urinary pheromone preference tests. Before urine collection, ovariectomized females were injected s.c. with EB (0.5 μg in 0.05 mL sesame oil, Sigma–Aldrich) followed 2 days later by a s.c. injection of P (830 μg in 0.05 mL sesame oil, Sigma–Aldrich) (Bonthuis et al. 2011). Urine was collected from females 3–6 h after the P injection when they were in behavioral estrus. Urine was collected separately from individual male and female mice while they were housed for 4 h into a metabolic chamber with free access to water but not food. Urine from each sex was then pooled and stored in 1-mL aliquots at −80 °C.
Chemosensory preference testing
All olfactory preference tests were carried out in a custom built testing box (Kunkhyen et al. 2017) under red light during the dark phase of the light:dark cycle. This plastic box (26.5 L × 20 W × 30 H cm) contained two 1-cm diameter circular odor ports on one side of the box spaced 17 cm apart. Urinary odors (20 µL) were placed on filter paper located 1 cm behind each odor port so as to allow mice to make nasal contact with the odor stimulus. This provided subjects access to the nonvolatile components of the urinary stimuli being presented. The duration of nose-poke behavior directed to each odor port was monitored by infrared beams at each odor port connected to an Arduino UNO board and software. The location of IMU and EFU was counterbalanced across testing days to prevent any side bias.
One day before the start of behavior testing, all females were habituated to the testing box for 30 min with access to the odor ports in the absence of odor stimuli. Ovary intact females were then given a series of pheromone preference tests on different days of the estrous cycle (Experiment 1). Ovariectomized females were tested on different days, first without any hormone replacement and then on separate days after a sequence of different treatments with E2 and P (Experiment 2). On each day of testing, female subjects were habituated in their home cage to the testing room for at least 3 h before odor preference tests began. Females were then placed in the testing box with access to the odor ports blocked for an initial 20 min. At the start of each 10-min test, urinary odors (20 µL) were placed on filter paper behind the odor ports, the barrier to the odor ports was removed, and the total duration of nose pokes into each odor port was recorded using the Arduino microcontroller. The total time spent investigating each odor port was determined. At the end of testing, females were returned to their home cage.
Experiment 1: Chemosensory preference testing in ovary-intact female mice
Sexually naïve, ovary-intact female mice (n = 10) were tested for their preference to investigate IMU versus EFU at 2 different stages of the estrous cycle. Vaginal lavages were taken daily in order to monitor females’ ovarian cycle (McLean et al. 2012; Dey et al. 2015). Distilled water (20 µL) was applied to the vagina using a pipette, and the resulting suspension was placed to dry on gelatin-coated microscope slides on a slide warmer for 45 min. Cells were then stained with 0.25% cresyl violet and examined using a light microscope. The murine estrous cycle can be divided into 4 separate stages (estrus, metestrus, diestrus, and proestrus) that reflect changes in circulating gonadal hormones (Wood et al. 2007). Females were categorized as being in estrus when the majority of the cells present in the lavage were cornified squamous epithelial cells. Females were categorized as being in nonestrus when either >90% of the cells present in the lavage were leukocytes (diestrus) or when >50% of cells present were leukocytes (metestrus) (Inderdeo et al. 1996; McLean et al. 2012) (Figure 1). Proestrous vaginal cytology (exclusively nucleated epithelial cells) was rarely seen in our females. Females were tested for urinary pheromone preference when they were either in an estrous or nonestrous condition, approximately 3 h after lavages were taken. Females were tested on 4 separate days: twice while in the estrous condition and twice while in the nonestrous condition according to the chemosensory testing protocol described above. Investigation times at each odor port were averaged for each mouse across the 2 tests in the estrous condition and across the 2 tests in the nonestrous condition.
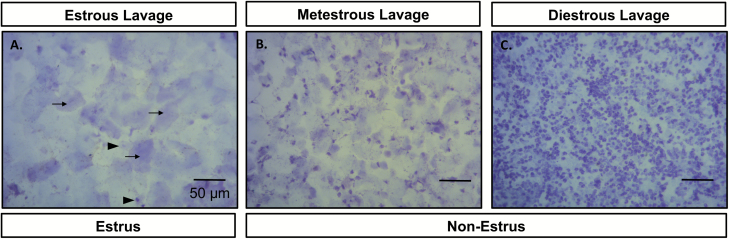
Figure 1.
Representative photomicrographs of cells from vaginal lavages of ovary intact female mice in either an estrous condition (A, estrous lavage: black arrows point to representative cornified squamous epithelial cells, black arrow heads point to representative nucleated epithelial cells) or 1 of 2 nonestrous conditions (B, metestrous lavage; C, diestrous lavage). Females were categorized as being in an estrous condition when the majority of the cells present in the lavage were cornified squamous epithelial cells. Females were categorized as being in a nonestrous condition when either >90% of the cells present in the lavage were leukocytes (diestrus) or when >50% of cells present were leukocytes (metestrus).
Experiment 2: Effects of mating experience and hormone replacement on chemosensory preferences of ovariectomized females
Ovariectomized female mice (n = 18) were tested for their preference to investigate IMU versus EFU. One group of females (n = 9) was sexually naïve at the onset of pheromone preference testing. A second group of females (n = 9) was given mating experience with a stud male before pheromone preference testing. These latter females were brought into behavioral estrus 7 days after ovariectomy with s.c. injections of EB followed 2 days later with P whereupon they were immediately paired overnight with a stud male. This was done on 2 separate occasions, and females in which seminal plugs were found in the vagina the next morning on at least one occasion were deemed to be sexually experienced. All females received a series of 5 preference tests for IMU versus EFU after receiving different ovarian hormone treatments beginning 3 weeks after ovariectomy. In the case of the mating-experienced group, the first pheromone preference test was given at least 1 week after the last P injection had been administered to provide mating experience. Ovariectomized females in both groups were given no ovarian hormones before the first pheromone preference (Test 1: no hormone). The following day, females received a s.c. injection of EB (0.5 μg in 0.05 mL sesame oil) whereupon they were tested for pheromone preference 4 h later (Test 2: EB injection). Two days later females were injected s.c. with P (830 μg in 0.05 mL sesame oil) whereupon they were tested again 4 h later (Test 3: EB + P). The next day all females were implanted s.c. with a silicone SILASTIC capsule containing E2 under the skin of the back of the neck under 2% isoflurane anesthesia. The E2 was diluted 1:1 with cholesterol in these capsules (inner diameter 1.57 mm; outer diameter 2.41 mm; length 5 mm) (DiBenedictis et al. 2012). Females were allowed to recover for 1 week, and were then tested for pheromone preference once again (Test 4: E2 capsule) and then again the following day, 4 h after females received a s.c. P injection (500 μg) (Test 5: E2 capsule + P injection). A timeline for this experiment can be found in Figure 2. Previous studies looking at the expression of lordosis behavior in ovariectomized female mice have used either a s.c. EB injection followed 2 days later by a s.c. P injection (Bonthuis et al. 2011; McCarthy et al. 2017) or long-term s.c. implantation of an E2 capsule sequenced with a s.c. P injection to induce behavioral estrus (Keller et al. 2006; Brock et al. 2011; DiBenedictis et al. 2012). We tested chemosensory preferences in ovariectomized female mice using these 2 different methods of steroid hormone delivery. Note that we did not administer s.c. injections of sesame oil vehicle to additional control subjects on the presumption that any minor stress resulting in our hormone-primed subjects that received s.c. injections would have dissipated over the 3–4 h period between hormone injections and chemosensory preference testing.
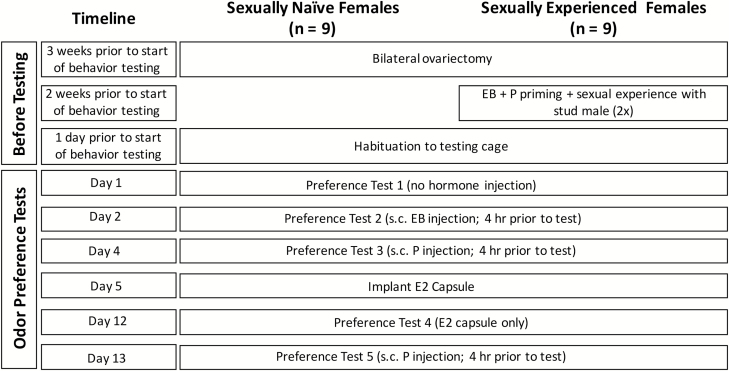
Figure 2.
Timeline of procedures carried out in Experiment 2. Abbreviations: EB, estradiol benzoate; P, progesterone; E2, estradiol.
Statistical analysis
For both experiments, pheromone preference test results were analyzed with paired, 2-tailed t-tests to determine if subjects preferred to investigate IMU versus EFU. The total time that subjects spent at either of the 2 odor ports was also compared using paired 2-tailed t-tests between the estrous and nonestrous conditions (Experiment 1) and across the 5 different hormone replacement conditions using a 1-way repeated measures ANOVA (Experiment 2). The total investigation time was also analyzed in both experiments to determine whether subjects’ interest in pheromonal stimuli varied as a function of endocrine status and/or waned with repeated testing. Statistical analysis was carried out using SigmaPlot11 software (Systat Software).
Results
Experiment 1: Chemosensory preferences of ovary-intact female mice
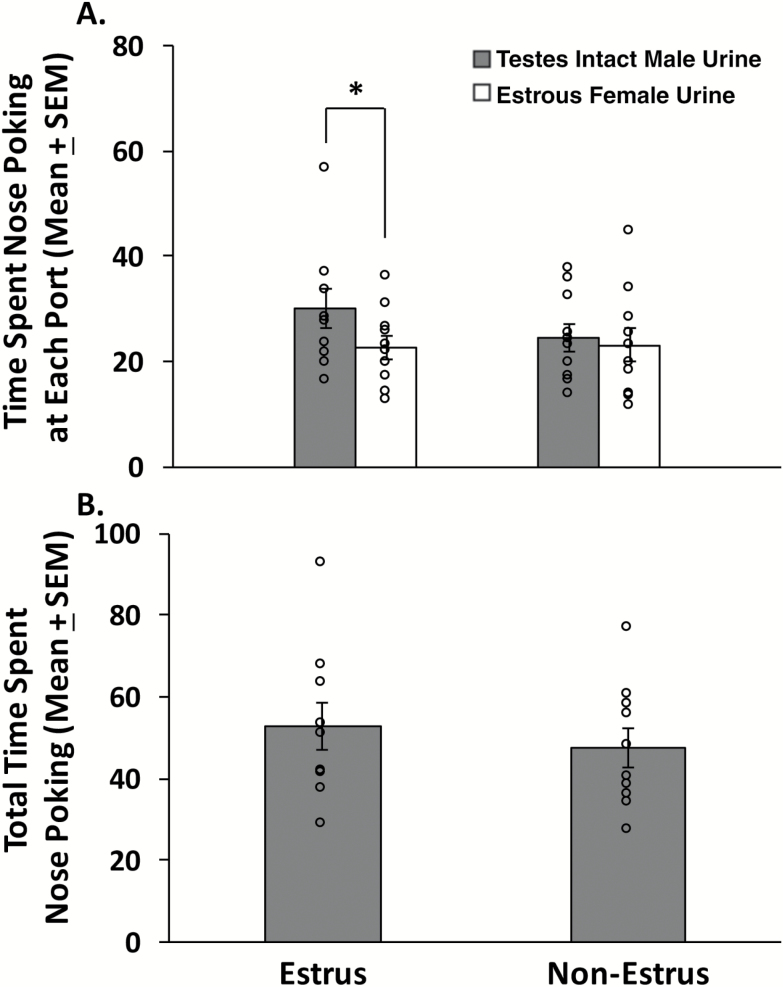
Sexually naïve, ovary-intact females showed a significant preference for IMU versus EFU (Figure 3A), provided they were in vaginal estrus (t9 = 3.326, P = 0.009). However, when these same females were not in vaginal estrus no significant preference for either urinary odor was observed (t9 = 0.383, P = 0.711). There was no significant difference between estrous and nonestrous conditions in the total amount of time that females spent investigating either odor port (t9 = 0.625, P = 0.548) (Figure 3B), suggesting that subjects remained active and attentive to the olfactory choices provided even when in the nonestrous condition.
Figure 3.
Preference for testes-intact male urine (IMU) compared to estrous female urine (EFU) in sexually naïve, ovary-intact female mice (n = 10). (A) The time (in seconds) that females spent investigating the odor ports containing IMU versus EFU was recorded when subjects were either in vaginal estrus or nonestrus. Females were tested for pheromone preference twice while in estrus and in nonestrus, and the time spent investigating each odor port was averaged across the 2 tests given under each cycle stage. (B) The total time spent investigating either odor port (IMU + EFU investigation times in seconds) while female subjects were in either estrus or nonestrus. Data are expressed as the mean ± SEM, while the circles represent the individual data points for each mouse. *P < 0.05 comparison of investigation times at each odor port when subjects were in vaginal estrus.
Experiment 2: Effects of mating experience and hormone replacement on chemosensory preferences of ovariectomized females
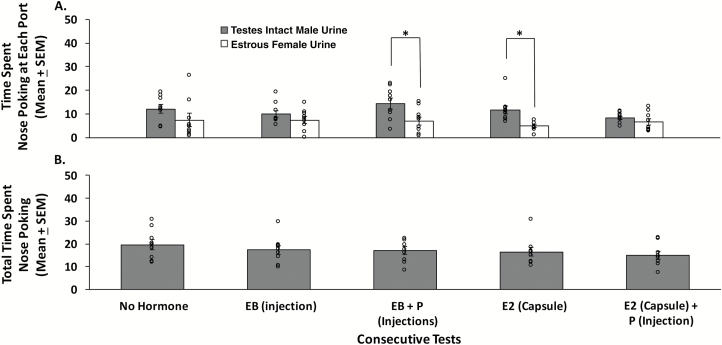
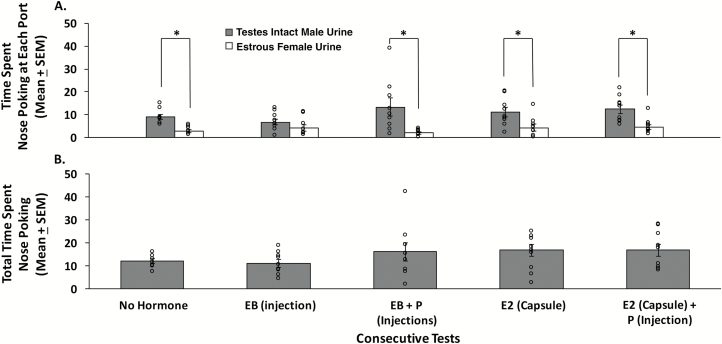
Ovariectomized, sexually naïve females showed a significant preference to investigate IMU as opposed to EFU only after they either received injections of EB sequenced 2 days later with P (t8 = −2.740, P = 0.025) or treatment for 1 week with a s.c. capsule containing E2 (t8 = −3.778, P = 0.005) (Figure 4A). By contrast, ovariectomized, sexually naïve females failed to prefer IMU over EFU when tested without any hormone treatment, 4 h after a single injection of EB, or after s.c. implantation of an E2 capsule followed 1 day later by an injection of P. The total time spent investigating either odor port (time spent investigating IMU + time spent investigating EFU) was equivalent regardless of the hormone treatments received (F4,44 = 0.703, P = 0.596) (Figure 4B), suggesting that subjects remained active and attentive to the odorant stimuli presented, regardless of whether or not they preferred investigating one over the other in particular tests.
Figure 4.
The effect of different ovarian hormone treatments on preference for testes-intact male urine (IMU) compared to estrous female urine (EFU) in ovariectomized, sexually naïve female mice (n = 9). (A) Pheromone preference as determined by the time (in seconds) spent investigating the odor ports containing IMU versus EFU in a series of 5 olfactory preference tests given after treatment with different ovarian hormones. (B) The total time spent investigating either odor port (IMU + EFU investigation times in seconds) during each of the 5 olfactory preference tests. Data are expressed as the mean ± SEM, while the circles represent individual data points for each mouse. *P < 0.05 for comparisons of investigation times at each odor port on different test days. Abbreviations: EB = estradiol benzoate; P = progesterone; E2 = estradiol.
A second group of ovariectomized female mice given sexual experience with a stud male before odor preference testing showed a significant preference for IMU compared to EFU in the absence of ovarian hormone treatment (t8 = −5.002, P = 0.001), after an EB injection followed 2 days later by a P injection (t8 = −2.999, P = 0.017), after the presence of an implanted E2 capsule for 1 week (t8 = −2.714, P = 0.026), and after one P injection given 8 days after implantation of an E2 capsule (t8 = −5.193, P < 0.001) (Figure 5A). By contrast, a single EB injection given 4 h before testing induced only a nonsignificant trend toward a preference for IMU versus EFU (t8 = −1.211, P = 0.260). Comparison of the total time spent investigating either odor port showed no effect of the different hormone treatments (F4,44 = 1.165, P = 0.345) (Figure 5B), again, suggesting that subjects remained active and attentive to the odorant stimuli presented, regardless of whether or not they preferred investigating one over the other in particular tests.
Figure 5.
The effect of different ovarian hormone treatments on preference for testes-intact male urine (IMU) compared to estrous female urine (EFU) in ovariectomized female mice that had previously received mating experience with a stud male (n = 9). (A) Pheromone preference as determined by the time (in seconds) spent investigating the odor port containing IMU versus EFU in a series of 5 olfactory preference tests after treatment with different ovarian hormones. (B) The total time spent investigating either odor port (IMU + EFU investigation times in seconds) during each of the 5 olfactory preference tests. Data are expressed as the mean ± SEM, while the circles represent individual data points for each mouse. *P < 0.05 for comparisons of investigation times at each odor port on different test days. Abbreviations: EB = estradiol benzoate; P = progesterone; E2 = estradiol.
Discussion
Female mice that lacked previous mating experience required exposure to ovarian hormones to display a preference for IMU over EFU. Thus in Experiment 1, ovary-intact females which had not mated previously with a male only preferred to investigate IMU over EFU when they were in vaginal estrus. Likewise, in Experiment 2 sexually naïve females that had been ovariectomized showed no preference for male urinary pheromones in the absence of treatment with ovarian hormones (Test 1). This group only showed a significant preference to investigate male urinary odors after a single EB injection followed 2 days later by a P injection (4 h before Test 3) and after 7 days of continuous E2 exposure via a s.c. SILASTIC capsule (Test 4). These same sexually naïve ovariectomized females failed to show a significant preference for male pheromones when tested only 4 h after an EB injection (Test 2) or 4 h after a P injection (Test 5), with an E2 capsule previously having been in place for 8 days. It is possible that in the former case the absence of a significant preference for male pheromones reflected the inadequate passage of time (only 4 h) needed for a genomic action of E2 in the nervous system. In the latter case, there was a trend for E2-primed, P-treated females to prefer male pheromones that missed statistical significance. What is clear from both studies using sexually naïve females is that a male pheromone preference was not seen in the absence of estrous stage concentrations of circulating ovarian hormones.
In Experiment 2, ovariectomized female mice that previously received mating experience showed a significant preference for IMU over EFU even in the absence of hormone treatment. In subsequent tests, given after several different regimens of ovarian hormones, these same mating-experienced females usually continued to show a significant preference to investigate IMU over EFU. The only exception was in Test 2 given 4 h after a single injection of EB in which there was only a nonsignificant trend for these females to prefer IMU. Our results indicate that priming with ovarian hormones may not be required for female mice with prior mating experience to display a preference for male urinary pheromones. The results obtained suggest that the interactive actions of ovarian hormones and genital-somatosensory inputs resulting from the receipt of mounts and intromissions from a male exert a long-lasting change in the responsiveness of the olfactory input circuit that processes pheromones such that mating experienced females prefer to seek out male pheromones even in the absence of ovarian hormone action in the forebrain.
In the current experiments, female mice were allowed nasal access to the heavy, nonvolatile as well as the lighter, volatile components of urine from testes-intact males. The results of Experiment 1 corroborate a previous study (Dey et al. 2015) in which sexually naïve female mice preferred to investigate either heavy molecular weight pheromones derived from urine of testes-intact males or recombinant MUPs, provided the females were in vaginal estrus as opposed to diestrus. This outcome contrasts with another early study (Scott and Pfaff 1970) in which ovary-intact female mice that lacked any prior mating experience preferred to investigate volatile urinary odors from testes-intact as opposed to castrated males regardless of whether they were in vaginal estrus or diestrus. This latter outcome may reflect the fact that volatile urinary pheromones are likely detected and processed via the main as opposed to accessory olfactory system (Baum and Kelliher 2009). Perhaps ovarian steroids exert no effect on relevant segments of the main olfactory system that process the sexually relevant features of pheromones. Further studies are needed to investigate the role of ovarian hormones on modulating the activity of different segments of the main olfactory system. We observed that ovary-intact female mice preferred to investigate male versus female urine on the day of estrus, which corresponds to the period around the time of ovulation when there is large surge in circulating E2 (Wood et al. 2007) followed by a smaller peak in circulating P (Walmer et al. 1992). Female mice are also most receptive to mounts from a male during vaginal estrus. Our results contrast with those of a previous study (Nomoto and Lima 2015) that compared nasal investigation directed by ovary-intact female mice toward the body of a male versus a female mouse. Those investigators reported that ovary-intact, sexually naive females preferred to investigate the body of a male significantly more than a female, regardless of whether they were in vaginal proestrus, estrus, or diestrus. The discrepancies between the 2 studies could reflect the use of stimuli from different mouse strains (BALB/c by Nomoto and Lima vs. C57BL/6J in the current experiments) and/or differences in the sources of male and female pheromones used in the 2 studies (whole body vs. urine).
A study using ovariectomized sexually experienced rats without hormone treatment found that these females showed no preference for volatile odors emitted from the body of testes-intact males compared to both castrated male and estrous female odors, whereas they showed a strong preference for testes-intact male odors when treated with EB and P (Xiao et al. 2004). This outcome in the rat differs from the results of our Experiment 2 in which sexually experienced female mice showed a strong preference to seek out male urinary pheromones even after ovariectomy and in the absence of any treatment with ovarian hormones. To our knowledge, there have been no other studies in female mice that examined the preference to investigate nonvolatile male urinary odors in sexually naïve compared to sexually experienced animals which were tested in the presence or absence of ovarian hormones. It is possible that the discrepancy between our study and previous studies in rats reflects a species difference in how ovarian hormones affect odor preference before and after sexual experience. It is also possible that procedural differences (in the current study females were exposed to both the heavy, nonvolatile plus the volatile components of urine whereas in Xiao (2004) subjects were exposed only to volatile chemosignals) could explain the different results.
The results of our study using sexually naïve ovariectomized female mice also contrasts with those of a previous study by Moncho-Bogani et al. (2004) which found that chemically naïve ovariectomized female mice preferred to investigate soiled bedding from testes-intact males compared to females in the absence of treatment with ovarian hormones and also after treatment with either EB alone, P alone, or EB + P. Female subjects in that study were given different hormonal treatments and then initially tested for their preference to investigate 2 different sources of female soiled bedding and then later tested on the same day for their preference to investigate male versus female soiled bedding. The female odor cues were pooled from the test subjects’ home cage bedding (Moncho-Bogani et al. 2004), making these odors very familiar to the subjects. It is possible that familiarity with the female odors may have enhanced female subjects’ preference for male odors, thereby masking any effects of the different hormone treatments. The female subjects in that previous study may not have been displaying a consistent preference for male odors regardless of hormone treatment, but rather a preference for novel pheromonal odors which happened to be derived from males. In the current study, we also tested preferences for urinary pheromones repeatedly in the same female subjects under each of the different hormone conditions. The females used in Moncho-Bogani et al. (2004) had not been previously exposed to any male urinary cues before testing and were only tested once in a specific hormone condition. It is possible that the repeated testing design of our experiment affected odor preference behavior in later tests compared to earlier ones. Future studies could randomize the order of testing in different hormone conditions to obviate this confound.
In ovary-intact females, there is a large peak of circulating P during diestrus and a smaller peak of P 2 days earlier around the time of estrus (Walmer et al. 1992). While we did not measure circulating levels of P in our ovary-intact females at the time of vaginal estrus, these females preferred to investigate IMU over EFU during this period of the estrous cycle when the smaller peak in circulating levels of P was predicted by Walmer et al. (1992). Using an in vitro preparation, the responsiveness of VNO sensory neurons in female mice to isolated MUPs from male urine was decreased when P was added to the medium, but the activity of these neurons in response to actual male urine was not decreased after the same P treatment (Dey et al. 2015). It would be interesting to know the activity of VNO sensory neurons in response to male urinary cues after sequential E2 followed by P treatments that mimic the concentrations of these 2 hormones just before vaginal estrus, which was when our results indicate that female mice exhibit the strongest preference for testes-intact male urine. In Experiment 2, both sexually experienced and sexually naïve ovariectomized females preferred to investigate testes-intact male urine after s.c. injection with EB followed 2 days later by a s.c. injection of P. Thus, maximal elevations in plasma P, if anything, worked to facilitate as opposed to inhibit females’ interest in male urinary pheromones. Treatment of ovariectomized female mice (Moncho-Bogani et al. 2004) and rats (Xiao et al. 2004; Hosokawa and Chiba 2007) with P in other studies also did not inhibit subjects’ preference to investigate testes-intact male chemosignals. Taken together, these results suggest that P normally acts in E2-primed females to augment their motivation to seek out male pheromones as opposed to suppressing females’ interest in these opposite-sex chemosignals, as was previously suggested by Dey et al. (2015).
In conclusion, female mice lacking mating experience require prior exposure to estradiol (typically followed by progesterone) to prefer investigating male over female urinary pheromones. However, the receipt of mating stimulation from a stud male, which occurs concurrently with the actions of ovarian hormones in the female’s forebrain, induces neural plasticity leading to a long-lasting male-oriented pheromone preference without any further hormonal stimulation.
Funding
This research was supported by the National Institute on Deafness and Other Communication Disorders [DC008962 to J.A.C.].
Acknowledgments
We thank Drs Wayne Korzan and Tenzin Kunkhyen for constructing the odor preference test box.
References
- Baum MJ, Kelliher KR. 2009. Complementary roles of the main and accessory olfactory systems in mammalian mate recognition. Annu Rev Physiol. 71:141–160. [DOI] [PubMed] [Google Scholar]
- Bonthuis PJ, Patteson JK, Rissman EF. 2011. Acquisition of sexual receptivity: roles of chromatin acetylation, estrogen receptor-alpha, and ovarian hormones. Endocrinology. 152:3172–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock O, Baum MJ, Bakker J. 2011. The development of female sexual behavior requires prepubertal estradiol. J Neurosci. 31:5574–5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamero P, Leinders-Zufall T, Zufall F. 2012. From genes to social communication: molecular sensing by the vomeronasal organ. Trends Neurosci. 35:597–606. [DOI] [PubMed] [Google Scholar]
- Cherian S, Wai Lam Y, McDaniels I, Struziak M, Delay RJ. 2014. Estradiol rapidly modulates odor responses in mouse vomeronasal sensory neurons. Neuroscience. 269:43–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. 2005. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 46:647–660. [DOI] [PubMed] [Google Scholar]
- de Castilhos J, Forti CD, Achaval M, Rasia-Filho AA. 2008. Dendritic spine density of posterodorsal medial amygdala neurons can be affected by gonadectomy and sex steroid manipulations in adult rats: a Golgi study. Brain Res. 1240:73–81. [DOI] [PubMed] [Google Scholar]
- Dey S, Chamero P, Pru JK, Chien MS, Ibarra-Soria X, Spencer KR, Logan DW, Matsunami H, Peluso JJ, Stowers L. 2015. Cyclic regulation of sensory perception by a female hormone alters behavior. Cell. 161:1334–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBenedictis BT, Ingraham KL, Baum MJ, Cherry JA. 2012. Disruption of urinary odor preference and lordosis behavior in female mice given lesions of the medial amygdala. Physiol Behav. 105:554–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DA. 1970. Induction of estrus in female mice: estrogen-progesterone interactions. HomBehav. 1: 299–304. [Google Scholar]
- Fata JE, Chaudhary V, Khokha R. 2001. Cellular turnover in the mammary gland is correlated with systemic levels of progesterone and not 17beta-estradiol during the estrous cycle. Biol Reprod. 65:680–688. [DOI] [PubMed] [Google Scholar]
- Halem HA, Cherry JA, Baum MJ. 1999. Vomeronasal neuroepithelium and forebrain Fos responses to male pheromones in male and female mice. J Neurobiol. 39:249–263. [PubMed] [Google Scholar]
- Hosokawa N, Chiba A. 2007. Effects of sexual experience on conspecific odor preference and male odor-induced activation of the vomeronasal projection pathway and the nucleus accumbens in female rats. Brain Res. 1175:66–75. [DOI] [PubMed] [Google Scholar]
- Inderdeo DS, Edwards DR, Han VK, Khokha R. 1996. Temporal and spatial expression of tissue inhibitors of metalloproteinases during the natural ovulatory cycle of the mouse. Biol Reprod. 55:498–508. [DOI] [PubMed] [Google Scholar]
- Ismail N, Garas P, Blaustein JD. 2011. Long-term effects of pubertal stressors on female sexual receptivity and estrogen receptor-α expression in CD-1 female mice. Horm Behav. 59:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse PD, Khokha R. 2010. Progesterone induces adult mammary stem cell expansion. Nature. 465:803–807. [DOI] [PubMed] [Google Scholar]
- Keller M, Douhard Q, Baum MJ, Bakker J. 2006. Destruction of the main olfactory epithelium reduces female sexual behavior and olfactory investigation in female mice. Chem Senses. 31:315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. 1981. Connections of the corticomedial amygdala in the golden hamster. I. Efferents of the “vomeronasal amygdala”. J Comp Neurol. 197:81–98. [DOI] [PubMed] [Google Scholar]
- Kunkhyen T, McCarthy EA, Korzan WJ, Doctor D, Han X, Baum MJ, Cherry JA. 2017. Optogenetic activation of accessory olfactory bulb input to the forebrain differentially modulates investigation of opposite versus same-sex urinary chemosignals and stimulates mating in male mice. eNeuro. 4:ENEURO.0010-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroche J, Gasbarro L, Herman JP, Blaustein JD. 2009a. Enduring influences of peripubertal/adolescent stressors on behavioral response to estradiol and progesterone in adult female mice. Endocrinology. 150:3717–3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroche J, Gasbarro L, Herman JP, Blaustein JD. 2009b. Reduced behavioral response to gonadal hormones in mice shipped during the peripubertal/adolescent period. Endocrinology. 150:2351–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy EA, Kunkhyen T, Korzan WJ, Naik A, Maqsudlu A, Cherry JA, Baum MJ. 2017. A comparison of the effects of male pheromone priming and optogenetic inhibition of accessory olfactory bulb forebrain inputs on the sexual behavior of estrous female mice. Horm Behav. 89:104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean AC, Valenzuela N, Fai S, Bennett SA. 2012. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp. 67:e4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncho-Bogani J, Lanuza E, Lorente MJ, Martinez-Garcia F. 2002. Attractive properties of sexual pheromones in mice: inate or learned? Physiol Behav. 77:167–176. [DOI] [PubMed] [Google Scholar]
- Nomoto K, Lima SQ. 2015. Enhanced male-evoked responses in the ventromedial hypothalamus of sexually receptive female mice. Curr Biol. 25:589–594. [DOI] [PubMed] [Google Scholar]
- Pardo-Bellver C, Cádiz-Moretti B, Novejarque A, Martínez-García F, Lanuza E. 2012. Differential efferent projections of the anterior, posteroventral, and posterodorsal subdivisions of the medial amygdala in mice. Front Neuroanat. 6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasia-Filho AA, Fabian C, Rigoti KM, Achaval M. 2004. Influence of sex, estrous cycle and motherhood on dendritic spine density in the rat medial amygdala revealed by the Golgi method. Neuroscience. 126:839–847. [DOI] [PubMed] [Google Scholar]
- Restrepo D, Arellano J, Oliva AM, Schaefer ML, Lin W. 2004. Emerging views on the distinct but related roles of the main and accessory olfactory systems in responsiveness to chemosensory signals in mice. Horm Behav. 46:247–256. [DOI] [PubMed] [Google Scholar]
- Ring JR. 1944. The estrogne-progesterone induction of sexual receptivity in the spayed female mouse. Endocrinology. 34: 269–275. [Google Scholar]
- Roberts SA, Davidson AJ, McLean L, Beynon RJ, Hurst JL. 2012. Pheromonal induction of spatial learning in mice. Science. 338:1462–1465. [DOI] [PubMed] [Google Scholar]
- Roberts SA, Simpson DM, Armstrong SD, Davidson AJ, Robertson DH, McLean L, Beynon RJ, Hurst JL. 2010. Darcin: a male pheromone that stimulates female memory and sexual attraction to an individual male’s odour. BMC Biol. 8:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JW, Pfaff DW. 1970. Behavioral and electrophysiological responses of female mice to male urine odors. Physiol Behav. 5:407–411. [DOI] [PubMed] [Google Scholar]
- Sorwell KG, Wesson DW, Baum MJ. 2008. Sexually dimorphic enhancement by estradiol of male urinary odor detection thresholds in mice. Behav Neurosci. 122:788–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson ML, Edwards DA. 1971. Experiential and strain determinants of the estrogen-progesterone induction of sexual receptivity in spayed female mice. Horm Behav. 2:299–305. [Google Scholar]
- Walmer DK, Wrona MA, Hughes CL, Nelson KG. 1992. Lactoferrin expression in the mouse reproductive tract during the natural estrous cycle: correlation with circulating estradiol and progesterone. Endocrinology. 131:1458–1466. [DOI] [PubMed] [Google Scholar]
- Wood GA, Fata JE, Watson KL, Khokha R. 2007. Circulating hormones and estrous stage predict cellular and stromal remodeling in murine uterus. Reproduction. 133:1035–1044. [DOI] [PubMed] [Google Scholar]
- Xiao K, Kondo Y, Sakuma Y. 2004. Sex-specific effects of gonadal steroids on conspecific odor preference in the rat. Horm Behav. 46:356–361. [DOI] [PubMed] [Google Scholar]