Fig. 1. FBXL19 mediates site-specific CBP ubiquitylation and degradation.
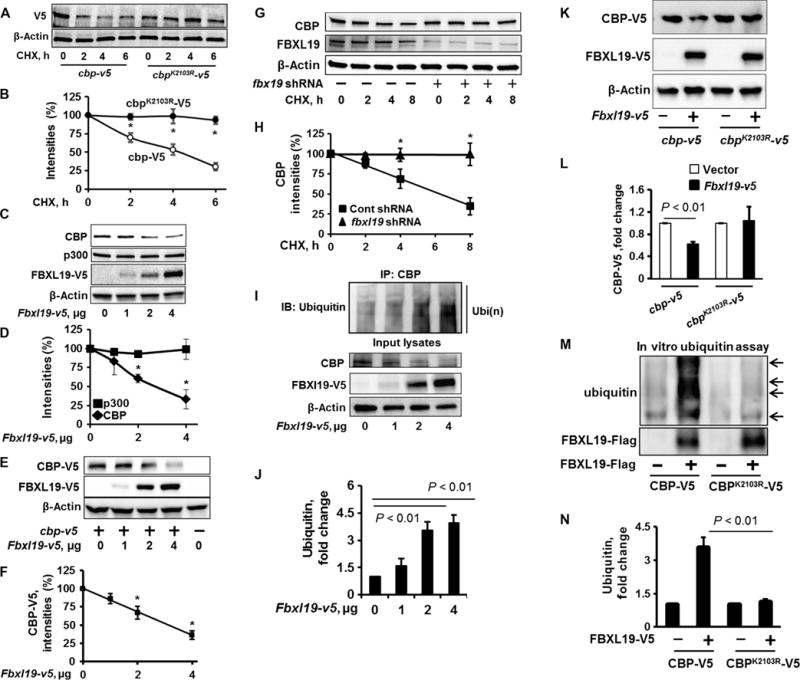
(A and B) MLE12 cells were transfected with the cbp-v5 or cbpK2103R-v5 plasmids. Forty-eight hours later, the cells were treated with CHX (20 μg/ml) for 0, 2, 4, or 6 hours. (A) Cell lysates were analyzed by Western blotting with antibodies against the indicated proteins. (B) Determination of relative protein abundance by densitometric analysis of Western blots with ImageJ software. (B) Data are means ± SEM of three independent experiments. *P < 0.01 by two-way analysis of variance (ANOVA) and post hoc Tukey’s test. (C and D) MLE12 cells were transfected with the indicated amounts of the Fbxl19-v5 plasmid. (C) Forty-eight hours later, cell lysates were analyzed by Western blotting with antibodies against the indicated proteins. (D) The relative amounts of p300 and CBP proteins were determined by densitometric analysis. Data are means ± SEM of three independent experiments. *P < 0.01 by two-way ANOVA and post hoc Tukey’s test. Comparison was made to the amount of CBP in cells that were not transfected with the Fbxl19-v5 plasmid. (E and F) MLE12 cells were transfected with the cbp-v5 and Fbxl19-v5 plasmids as indicated. (E) Forty-eight hours later, cell lysates were analyzed by Western blotting with antibodies against V5 and β-actin. (F) The relative amounts of CBP-V5 protein were determined by densitometric analysis. Data are means ± SEM of three independent experiments. *P < 0.01 by one-way ANOVA and post hoc Tukey’s test. Comparison was made to cells that were not transfected with the Fbxl19-v5 plasmid. (G and H) MLE12 cells were transfected with control (Cont) shRNA (−) or fbxl19-specific shRNA (+), and then cells were treated for the indicated times with CHX (20 μg/ml). (G) Cell lysates were analyzed by Western blotting with antibodies against CBP, FBXL19, and β-actin. (H) The relative amounts of CBP protein were determined by densitometric analysis. Data are means ± SEM of three independent experiments. *P < 0.01 by two-way ANOVA and post hoc Tukey’s test. Comparison was made to cells treated with control shRNA. (I and J) MLE12 cells were transfected with the indicated amounts of the Fbxl19-v5 plasmid. Forty-eight hours later, cell lysates were subjected to immunoprecipitation (IP) with an anti-CBP antibody and Western blotting analysis with an anti-ubiquitin antibody. Input lysates were analyzed by Western blotting with antibodies against CBP, V5, and β-actin. (J) The relative amounts of ubiquitylated CBP were determined by densitometric analysis. Data are means ± SEM of three independent experiments. P values were calculated by one-way ANOVA and post hoc Tukey’s test. Comparison was made to cells treated with control shRNA. IB, immunoblotting. Ubi(n), polyubiquitination. (K and L) MLE12 cells were transfected with the Fbxl19-v5, cbp-v5, or cbpK2103R-v5 plasmid, as indicated. (K) Cell lysates were then analyzed by Western blotting with antibodies against V5 and β-actin. (L) The relative amounts of CBP-V5 were determined by densitometric analysis. Data are means ± SEM of three independent experiments. P values were determined by the unpaired Student’s t test. (M and N) Troponin T (TnT)–synthesized CBP-V5 or CBPK2103R-V5 were incubated with a full complement of ubiquitylation reaction components with (+) or without (−) purified Flag-tagged FBXL19. (M) Reaction mixtures were subjected to Western blotting analysis with antibodies against ubiquitin and the Flag tag. The relative amounts of ubiquitylated CBP-V5 and CBPK2103R-V5 were determined by densitometric analysis. Data are means ± SEM of three independent experiments. P values were determined by one-way ANOVA and post hoc Tukey’s test. All Western blots are representative of at least three independent experiments.
