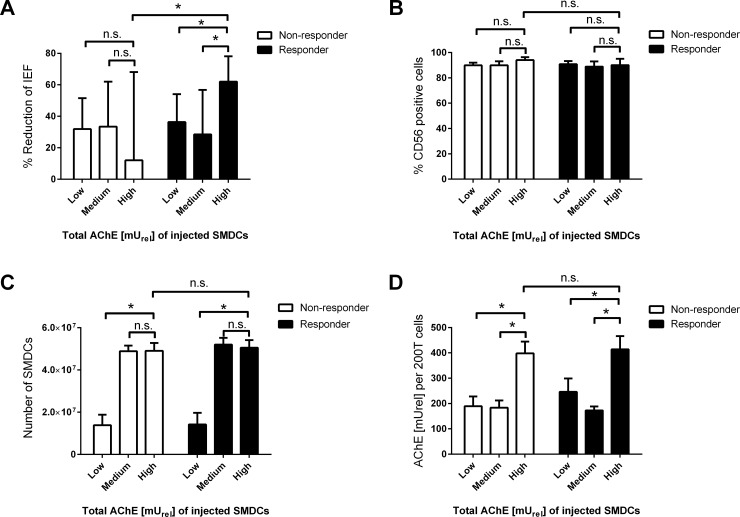
Fig 5. AChE activity and clinical efficacy.
Percent reduction of IEF from baseline to 6 months in responders receiving Low (n = 44), Medium (n = 15) or High (n = 11) and non-responders also receiving Low (n = 48), Medium (n = 22) and High (n = 9) total AChE activity SMDC batches (A). Percentage of CD56 positive cells (B), total number of cells (C), and AChE activity [mUrel] per 2*105 (200T) cells (D) in all SMDCs batches used for treatment of fecal incontinence in responder and non-responder patients according to total AChE activity. Data presented as mean and 95% confidence interval. Groups were compared by two-tailed unpaired t-test with Welch’s correction. A p-value below 0.05 was considered as significant (*) n.s.: not-significant.