Figure 1.
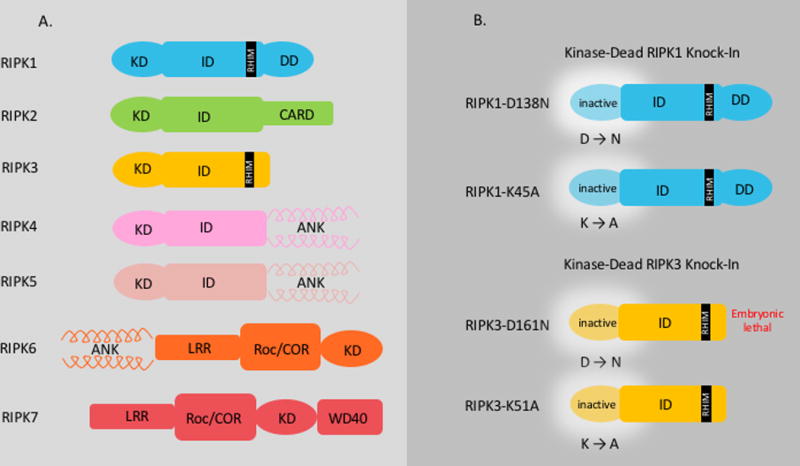
Panel A: The Receptor Interacting Kinase family is comprised of seven diverse members with a homologous Kinase Domain (KD). Each protein contains additional specific domains which are unique to the protein and dictate its function.
Panel B: The mutated Kinase-Dead Knock-In RIPK1 and RIPK3 mice that have been generated to study cell death are depicted. RIPK1-D138N: In order to render the kinase domain inactive, the catalytically important HKD (Asp-Phe-Gly) motif is altered. The conserved aspartate (D) at position 138 is mutated to asparagine (N) resulting in a “kinase-dead” protein. The mice are viable and fertile. RIPK1-K45A: A point mutation in the catalytic lysine (K) to alanine (A) in exon 3 of the RIPK1 gene eliminates all kinase activity resulting in a “kinase-dead” protein. These mice are viable and fertile. RIPK3 D161N: The aspartate (D) at position 161 in the catalytically important DFG (Asp-Phe-Gly) motif of the kinase domain of RIPK3 is mutated to an asparagine (N), rendering the protein catalytically inactive or “kinase-dead”. The mice generated with this mutation are not viable and die at E11.5. RIPK3-K51A: The lysine (K) at position 51 on the ATP-binding pocket is mutated to an alanine (A) resulting in “kinase-dead” RIPK3. These mice are viable and fertile. Abbreviations: ANK: Ankyrin Repeats, CARD: caspase activation and recruitment, KD: Kinase Domain, ID: Intermediary Domain, RHIM: RIP Homeotypic Interaction Motif, LRR: Leucine Rich Repeats, RIPK1: Receptor Interacting Kinase, roc/COR: Ras of complex proteins/C-terminal of Roc, WD40: WD40 Domain.
